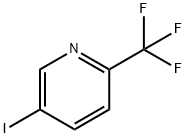
5-IODO-2-(TRIFLUOROMETHYL)PYRIDINE synthesis
- Product Name:5-IODO-2-(TRIFLUOROMETHYL)PYRIDINE
- CAS Number:873107-98-3
- Molecular formula:C6H3F3IN
- Molecular Weight:272.99
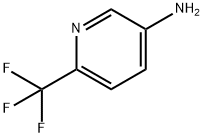
106877-33-2

873107-98-3
Step 1: Synthesis of 5-iodo-2-(trifluoromethyl)pyridine 6-(Trifluoromethyl)pyridin-3-amine (9.96 g, 0.062 mol) was dissolved in 5N HCl (70 mL) and cooled to -5 °C. A 30 mL aqueous solution of sodium nitrite (6.39 g, 0.093 mol) was slowly added dropwise while keeping the internal temperature below 5 °C. After the dropwise addition, the reaction was continued at -5 °C for 10 min. Subsequently, a 30 mL aqueous solution of KI (22.5 g, 0.136 mol) was added slowly dropwise while keeping the internal temperature below 10 °C. After completion of the dropwise addition, the reaction mixture was gradually warmed up to room temperature. Extraction was carried out by adding 250 mL of EtOAc, and the pH of the aqueous layer was adjusted to 11 by adding 50 mL of 6N NaOH. the organic and aqueous layers were separated, and the organic layer was washed with 120 mL of 0.3 M Na2S2O3 solution. The EtOAc layer was concentrated and the resulting concentrate was purified by silica gel column chromatography (eluent: hexane/EtOAc = 25/1) to give the title compound as a white solid (14.6 g, 87% yield). MS (ESI) calculated value: C6H3F3IN [M]+: 273.0; measured value: 274.0 [M+H]+. 1H NMR (400 MHz, CDCl3) δ 8.96 (s, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.47 (d, J = 8.2 Hz, 1H).

106877-33-2
274 suppliers
$8.00/1g

873107-98-3
72 suppliers
$60.00/100mg
Yield:873107-98-3 87%
Reaction Conditions:
Stage #1: 6-(trifluoromethyl)pyridin-3-aminewith hydrogenchloride;sodium nitrite in water at -5 - 5; for 0.166667 h;
Stage #2: with potassium iodide in water at -5 - 10;
Steps:
1.142.1 Step 1: 5-iodo-2-(trifluoromethyl)pyridine [0251] A solution of 6-(trifluoromethyl)pyridin-3-amine
Step 1: 5-iodo-2-(trifluoromethyl)pyridine [0251] A solution of 6-(trifluoromethyl)pyridin-3-amine (9.96 g, 0.062 mol) in 5N HC1 (70 mL) was cooled to -5°C and sodium nitrite (6.39 g, 0.093 mol) in 30 mL of water was added dropwise while maintaining the internal temperature below 5°C. After 10 min, KI (22.5 g, 0.136 mol) in 30 mL of water was added dropwise at -5°C while maintaining the internal temperature below 10°C over the course of the addition. The reaction mixture was warmed to rt and 250 mL of EtOAc was added. The pH of the aqueous layer was adjusted to 11 by the addition of 50 mL of 6N NaOH, the layers were separated, and the organic layer was washed with 120 mL of 0.3M Na2S203. The EtOAc layer was concentrated and the concentrate was purified by column chromatography over silica gel (hexane/ EtOAc =25/1) to afford the title compound as a white solid (14.6 g, 87%). MS (ESI) calcd for C6H3F3IN: 273.0; found: 274.0[M+H]. 1H NMR (400 MHz, CDC13) δ 8.96 (s, 1H), 8.22 (d, J = 8.2 Hz, 1H), 7.47 (d, J = 8.2 Hz, 1H).
References:
WO2015/187845,2015,A1 Location in patent:Paragraph 0251
436799-32-5
329 suppliers
$5.00/250mg

873107-98-3
72 suppliers
$60.00/100mg