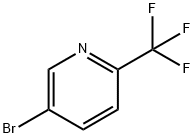
2-Trifluoromethyl-5-bromopyridine synthesis
- Product Name:2-Trifluoromethyl-5-bromopyridine
- CAS Number:436799-32-5
- Molecular formula:C6H3BrF3N
- Molecular Weight:225.99
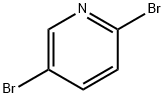
624-28-2
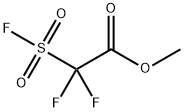
680-15-9

436799-32-5
Step 1: Synthesis of 5-bromo-2-(trifluoromethyl)pyridine To a stirred solution of 2,5-dibromopyridine (0.1 g, 0.421 mmol) in DMF (5.0 mL) was sequentially added methyl fluorosulfonyl difluoroacetate (0.405 g, 2.109 mmol) and cuprous iodide (0.401 g, 2.109 mmol). The reaction mixture was stirred at 100 °C for 12 h and the progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was cooled to room temperature, diluted with water and extracted with ethyl acetate (EtOAc). The organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to afford the crude product 5-bromo-2-(trifluoromethyl)pyridine (0.07 g, 73% yield) as a light yellow oil, which could be used in subsequent steps without further purification. Mass spectrum (MS): 227.28 [M++1].

624-28-2
720 suppliers
$5.00/5g

680-15-9
252 suppliers
$13.00/5g

436799-32-5
324 suppliers
$5.00/250mg
Yield:436799-32-5 73%
Reaction Conditions:
with copper(l) iodide in N,N-dimethyl-formamide at 100; for 12 h;
Steps:
30.1 Step 1: Synthesis of 5-bromo-2-(trifluoromethyl)pyridine
Step 1:
Synthesis of 5-bromo-2-(trifluoromethyl)pyridine
To a stirred solution of 2,5-dibromopyridine (0.1 g, 0.421 mmol) in DMF (5.0 mL) was added Methyl 2,2-difluoro-2-(fluorosulfonyl)acetate (0.405 g, 2.109 mmol) along with CuI (0.401 g, 2.109 mmol) and stirred at 100° C. for 12 h.
Completion of reaction was monitored by TLC.
Reaction mass was cooled to room temperature, diluted with water, extracted with EtOAc.
Organic portions were combined, dried over Na2SO4, evaporated under reduced pressure to obtain crude product 5-bromo-2-(trifluoromethyl)pyridine (0.07 g, 73%) as light yellow oil which was directly carry forward for next step.
MS: 227.28[M++1]
References:
Mankind Pharma Ltd.;Patil, Rakesh Ishwar;Verma, Jeevan;Shah, Dharmesh;Ali, Sazid;Bapuram, Srinivasa Reddy;Rai, Santosh Kumar;Kumar, Anil US2017/291910, 2017, A1 Location in patent:Paragraph 0577-0579
383-62-0
246 suppliers
$15.00/5 g
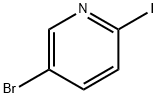
223463-13-6
382 suppliers
$5.00/1g

436799-32-5
324 suppliers
$5.00/250mg

223463-13-6
382 suppliers
$5.00/1g
81290-20-2
417 suppliers
$13.00/1g

436799-32-5
324 suppliers
$5.00/250mg
106877-33-2
268 suppliers
$8.00/1g

436799-32-5
324 suppliers
$5.00/250mg
2314-97-8
186 suppliers
$40.00/25 g

223463-13-6
382 suppliers
$5.00/1g
7440-66-6
0 suppliers
$14.00/5g

436799-32-5
324 suppliers
$5.00/250mg