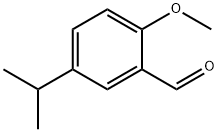
5-ISOPROPYL-2-METHOXYBENZALDEHYDE synthesis
- Product Name:5-ISOPROPYL-2-METHOXYBENZALDEHYDE
- CAS Number:85902-68-7
- Molecular formula:C11H14O2
- Molecular Weight:178.23
4132-48-3
112 suppliers
$11.00/1g

85902-68-7
33 suppliers
$47.29/5g
Yield:-
Reaction Conditions:
with sodium hydroxide;hexamethylenetetramine;magnesium sulfate in water;trifluoroacetic acid;
Steps:
4 EXAMPLE 4
EXAMPLE 4 To each of two-separate 22-L three-necked, round-bottomed reaction flasks equally equipped with thermometer, reflux condenser and mechanical stirrer, there were charged 1.3 Kg (7.4 moles) of 4-isopropylanisole (the product of Example 2) and 12.0 L of trifluoroacetic acid at ambient temperatures (ca. 20° C.) with constant agitation being maintained throughout the course of the entire addition step. This was then followed by the slow addition of 1.0 Kg (7.4 moles) of hexamethylenetetramine in small portions over a 50-minute period to the two well-stirred reaction mixtures now contained in each of the two-separate reaction flasks. The resulting reaction mixture in each flask then exothermed from 24° C. to 38° C. in each instance and was thereafter externally heated up to the reflux temperature of the reaction mixture at 81° C. The stirred and heated reaction mixture was next refluxed at this point for a period of 14 hours. Upon completion of this step, the trifluoroacetic acid solvent was removed from each of the two spent reaction mixtures via concentration under reduced pressure and each resulting residual oil was thereafter partitioned between 4 L of water and 4 L of hexanes. The pH of the two-phase system now contained in each flask was subsequently re-adjusted from a pH value of 0.5 to a value of pH 9.0 by adding thereto separate-3.8 L and 4.2 L portions of 6N aqueous sodium hydroxide solution, respectively. The basified two-phase systems were then allowed to settle and separate, and the two-separated aqueous layers were thereafter saved and subsequently washed with a fresh 2 L-portion of hexanes. The organic layers were next combined and subsequently backwashed with 3 L of water, followed by treatment with 200 g of activated charcoal and 400 g of anhydrous magnesium sulfate as drying agent. After removal of the latter two substances by means of suction filtration through celite, the resulting clear organic filtrate was subsequently concentrated in vacuo to give 2.0 Kg (72%) of crude aldehyde final product in the form of a dark residual oil. The latter material (1.8 Kg) was then purified by means of distillation in vacuo to ultimately afford 880 g (44%) of pure 2-methoxy-5-isopropylbenzaldehyde. The pure product was characterized by means of nuclear magnetic resonance (NMR) data. NMR Data: 1 H NMR(CD3 OD) δ1.05(d, 6H), 2.74(m, 1H), 3.75(s, 3H), 6.95(d, 1H), 7.35(dd, 1H), 7.35(dd, 1H), 7.5 1H, 10.23(s, 1H).
References:
US5294744,1994,A