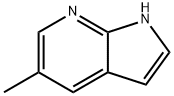
5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE synthesis
- Product Name:5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE
- CAS Number:824-52-2
- Molecular formula:C8H8N2
- Molecular Weight:132.16
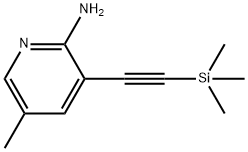
500903-95-7
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
The general procedure for the synthesis of 5-methyl-7-azaindole from 5-methyl-3-((trimethylmethylsilyl)alkynyl)pyridin-2-amine was as follows: 5-methyl-3-((trimethylsilyl)alkynyl)pyridin-2-amine (9.8 g, 48.0 mmol) and N-methylpyrrolidinone (50 mL) were added to a dry three-necked flask. Under ice bath conditions, sodium hydride (60% dispersed in mineral oil, 2.8 g, 59 mmol) was slowly added, followed by heating the reaction mixture to 100 degrees Celsius and holding for 10 minutes. Upon completion of the reaction, the mixture was cooled to room temperature and saturated ammonium chloride solution (50 mL) was slowly added to quench the reaction. Extraction was performed with ethyl acetate (50 mL x 3) and the organic phases were combined. The organic phase was washed sequentially with water (100 mL) and saturated brine (100 mL) and then dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness in a rotary evaporator to obtain the crude product. The crude product was recrystallized with ethyl acetate (10 mL) to give 5-methyl-7-azaindole (5.1 g, 38.6 mmol, 80% yield) as a white solid.

500903-95-7
16 suppliers
$155.00/50mg
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
103 suppliers
$25.00/50mg
Yield:824-52-2 80%
Reaction Conditions:
with sodium hydride in 1-methyl-pyrrolidin-2-one;mineral oil at 100; for 0.166667 h;Cooling with ice;
Steps:
1.3 Step 3
Compound 2 (9.8 g, 48.0 mmol) and N-methylpyrrolidone (50 mL) were added to a three-necked flask;Sodium hydride (60%, 2.8 g, 59 mmol) was added to the ice bath,100 degrees Celsius for 10 minutes. Cool to room temperature,Saturated ammonium chloride solution (50 mL) was added and extracted with ethyl acetate (50 mL x 3); the organic phases were combined, washed with water (100 mL) and saturated brine (100 mL), dried over sodium sulphate and filtered. The filtrate was spin dried to give the crude product, which was recrystallized from ethyl acetate (10 mL) to give white solid, compound 3 (5.1 g, 38.6 mmol, 80%).
References:
Kanghua (Shanghai) Drug Discovery Co., Ltd.;Ma Jingxiang CN107056781, 2017, A Location in patent:Paragraph 0008
![1H-Pyrrolo[2,3-b]pyridine, 5-methyl-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/918523-66-7.gif)
918523-66-7
2 suppliers
inquiry
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
103 suppliers
$25.00/50mg
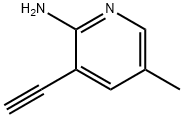
863479-77-0
11 suppliers
inquiry
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
103 suppliers
$25.00/50mg
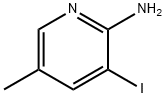
211308-79-1
81 suppliers
$28.00/250mg
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
103 suppliers
$25.00/50mg
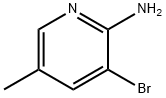
17282-00-7
332 suppliers
$5.00/1g
![5-METHYL-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/824-52-2.gif)
824-52-2
103 suppliers
$25.00/50mg