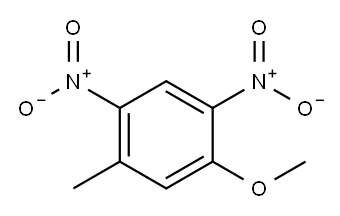
5-methyl-2,4-dinitroanisole synthesis
- Product Name:5-methyl-2,4-dinitroanisole
- CAS Number:3606-21-1
- Molecular formula:C8H8N2O5
- Molecular Weight:212.16
Yield:3606-21-1 95%
Reaction Conditions:
with potassium hydroxide at 20 - 30; for 0.5 h;
Steps:
2D Example 2D
To a suspension of l-fluoro-5-methyl-2,4-dinitro-benzene (100 g, 0.5 mol) in methanol (0.5 L) was added KOH (56 g, 0.1 mmol) in methanol (0.5 mL) drop wise at 25-30 °C. Then the resulting suspension was stirred at room temperature over a period of 30 minutes. The reaction mixture was quenched with water at 0-5 °C, the reaction mixture was filtered and dried under vacuum to obtain l-methoxy-5-methyl-2,4-dinitro-benzene as off-white solid (101 g, 95%).1H NMR (DMSO-d6, 300 MHz): 2.67 (s, 3H), 4.05 (s, 3H), 7.52 (s, 1H), 8.65 (s, 1H);LC-MS (ESI): 211.0 (M-H)
References:
WO2014/195896,2014,A1 Location in patent:Page/Page column 34-36
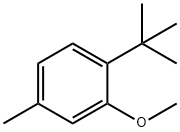
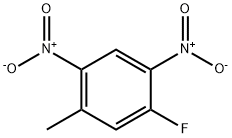
88-40-4
9 suppliers
inquiry

3606-21-1
6 suppliers
inquiry
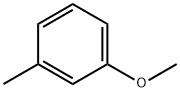
100-84-5
263 suppliers
$9.00/25g

3606-21-1
6 suppliers
inquiry
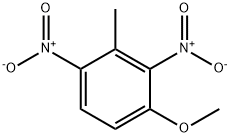
30366-26-8
1 suppliers
inquiry
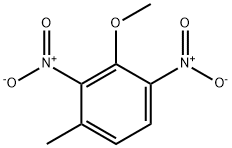
860734-16-3
0 suppliers
inquiry