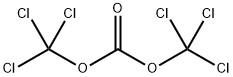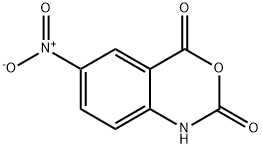
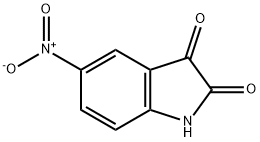
5-NITROISATOIC ANHYDRIDE synthesis
- Product Name:5-NITROISATOIC ANHYDRIDE
- CAS Number:4693-02-1
- Molecular formula:C8H4N2O5
- Molecular Weight:208.13
Yield:4693-02-1 75%
Reaction Conditions:
in tetrahydrofuran at 70; for 12 h;
Steps:
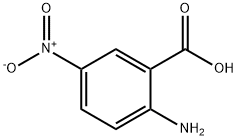
General procedure for isatoic anhydride synthesis 6-Bromo-2H-benzo[d][1,3]oxazine-2,4(1H)-dione (9b).
General procedure: A 500 mL single neck round-bottomed flask equipped with a football-shaped PTFE stirring bar (16 mm × 37 mm) was chargedwith 2-amino-5-bromobenzoic acid (10.0 g, 46.3 mmol,1.0 equiv) followed by the addition of tetrahydrofuran (230 mL,0.2 molar) and solid triphosgene (13.7 g, 46.3 mmol, 1.0 equiv)resulting in a suspension. The reaction vessel was placed into afitted metal heating mantle and the neck was equipped with a24/40 Liebig condenser. The suspension was stirred (500 rpm)and the heating mantle set to 70 °C. The suspension becamehomogenous before a white solid precipitated out after about30 minutes at 70 °C. The heterogeneous reaction mixture wasaged for 12 hours then cooled to room temperature (25 °C). Theslurry was poured into a 600 mL beaker equipped with overheadmechanical stirrer (PTFE 75 mm paddle) containing250 mL of deionized water. With vigorous stirring, the mixturebecame homogenous followed by precipitation of a pale whitesolid. The solid was collected by vacuum filtration on aBüchner funnel (7.6 cm diameter) with Whatman 1 filter paper(70 mm) and air pulled through for 5 minutes. The material was transferred to a 250 mL Erlenmeyer flask equipped with cylindricalstir bar and 50 mL of methanol was added. The slurrywas stirred for 10 minutes and then collected by vacuum filtration.The filter cake was dried under vacuum (0.1 mmHg at 25 °C) for 12 hours to afford 9b as a white powder (90% yield).
References:
Jentsch, Nicholas G.;Hume, Jared D.;Crull, Emily B.;Beauti, Samer M.;Pham, Amy H.;Pigza, Julie A.;Kessl, Jacques J.;Donahue, Matthew G. [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2529 - 2536]
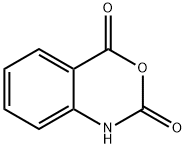
118-48-9
442 suppliers
$5.00/5 g

4693-02-1
118 suppliers
inquiry
616-79-5
351 suppliers
$8.00/5g

4693-02-1
118 suppliers
inquiry
611-09-6
202 suppliers
$6.00/1g

4693-02-1
118 suppliers
inquiry

124-38-9
134 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry
6293-83-0
161 suppliers
$6.00/250mg

4693-02-1
118 suppliers
inquiry