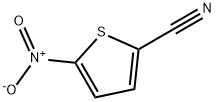
5-NITROTHIOPHENE-2-CARBONITRILE synthesis
- Product Name:5-NITROTHIOPHENE-2-CARBONITRILE
- CAS Number:16689-02-4
- Molecular formula:C5H2N2O2S
- Molecular Weight:154.15
![(NZ)-N-[(5-nitrothiophen-2-yl)methylidene]hydroxylamine](/CAS/20210305/GIF/6030-18-8.gif)
6030-18-8

16689-02-4
General procedure for the synthesis of 5-nitrothiophene-2-carbonitrile from the compound (CAS: 6030-18-8): oxime (1.0 mmol) and 2-nitrobenzenesulfonate (1.5 mmol) were dissolved in anhydrous dichloromethane (5.0 mL) under nitrogen protection in an oven-drying 50 mL round-bottomed flask fitted with a stirrer. The reaction mixture was stirred at room temperature for 5 min and then 1,8-diazabicycloundec-7-ene (DBU, 2.5 mmol) was slowly added dropwise over 2 min. Upon completion of the dropwise addition, the reaction mixture changed to a clarified homogeneous solution. The reaction process was monitored by thin layer chromatography (TLC). After complete consumption of the starting material, the reaction mixture was diluted with ethyl acetate and washed sequentially with water (2 × 5 mL) and brine (2 × 5 mL). The product was purified by column chromatography. The by-product Oxyma can be recovered by acidifying the aqueous layer followed by extraction with ethyl acetate. The recovered Oxyma can be used to regenerate Oxyma sulfonate, which in turn can be used in another batch reaction.
![(NZ)-N-[(5-nitrothiophen-2-yl)methylidene]hydroxylamine](/CAS/20210305/GIF/6030-18-8.gif)
6030-18-8
0 suppliers
inquiry

16689-02-4
135 suppliers
$8.00/1g
Yield:16689-02-4 83%
Reaction Conditions:
with (E)-ethyl 2-cyano-2-(2-nitrophenylsulfonyloxyimino)acetate;1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 20;Inert atmosphere;
Steps:
Representative procedure for nitrile synthesis
General procedure: In an oven-dried two-necked 50 mLround-bottomed flask, equipped with a stirring bar, a solution of the oxime(1.0 mmol) and 2-NO2-C6H4-SO3XY(1.5 mmol) dissolved in anhydrous CH2Cl2 (5.0 mL) wasplaced under the atmosphere of nitrogen. The reaction mixture was stirred atroom temperature for 5 min, then DBU (2.5 mmol) was added drop wise over 2 min.The reaction mixture became a clear homogeneous solution after addition of DBU.The reaction was monitored by TLC. The reaction mixture was diluted with EtOAcand washed with water (2×5 mL) followed by brine (2×5 mL) upon completeconsumption of the starting material. Product was purified by columnchromatography.Furthermore, the by-product Oxymacould be readily recovered by acidifying the aqueous layer, and then extractingwith ethyl acetate. The Oxyma thus recovered can then be reused to regeneratethe sulfonate ester of Oxyma, which can be further used for a separate batch ofreaction.
References:
Dev, Dharm;Palakurthy, Nani Babu;Kumar, Nitesh;Mandal, Bhubaneswar [Tetrahedron Letters,2013,vol. 54,# 33,p. 4397 - 4400] Location in patent:supporting information
![2-Thiophenecarboxaldehyde, 5-nitro-, oxime, [C(E)]-](/CAS/20211123/GIF/104416-19-5.gif)
104416-19-5
0 suppliers
inquiry

16689-02-4
135 suppliers
$8.00/1g
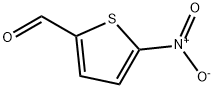
4521-33-9
160 suppliers
$10.00/5g

16689-02-4
135 suppliers
$8.00/1g
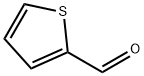
98-03-3
545 suppliers
$5.00/25g

16689-02-4
135 suppliers
$8.00/1g
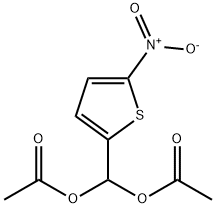
14289-24-8
9 suppliers
inquiry

16689-02-4
135 suppliers
$8.00/1g