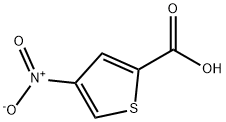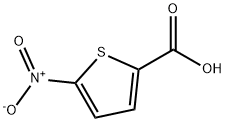
5-Nitrothiophene-2-carboxylic acid synthesis
- Product Name:5-Nitrothiophene-2-carboxylic acid
- CAS Number:6317-37-9
- Molecular formula:C5H3NO4S
- Molecular Weight:173.15
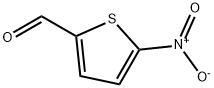
4521-33-9

6317-37-9
General procedure for the synthesis of 5-nitrothiophene-2-carboxylic acid from 5-nitrothiophene-2-carboxaldehyde: 5-nitrothiophene-2-carboxaldehyde (1.685 g, 10.72 mmol) was dissolved in dioxane (30 mL) with sulfamic acid (1.249 g, 12.87 mmol) and cooled down to 0 °C. Subsequently, an aqueous solution of sodium chlorite (1.940 g, 21.44 mmol) was added slowly and dropwise (14 mL). The reaction mixture was gradually warmed to room temperature and stirred continuously for 2 hours. A second batch of 5-nitrothiophene-2-carbaldehyde (1.966 g, 12.51 mmol) was treated at the same molar ratio under the same conditions. The two reaction mixtures were combined and extracted by partitioning with ethyl acetate and water. The organic phase was extracted twice with 5% NaHCO3 solution and discarded. The basic aqueous phase was adjusted to pH=2 with 2N HCl and extracted twice with ethyl acetate. The organic layers were combined, washed with brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give 5-nitrothiophene-2-carboxylic acid (Int.53) (3.6 g, 20.79 mmol, MS/ESI+ 173.9 [MH]+).

4521-33-9
160 suppliers
$10.00/5g

6317-37-9
198 suppliers
$5.00/1g
Yield:6317-37-9 98.5%
Reaction Conditions:
with bromine;anhydrous Sodium acetate;glacial acetic acid
Steps:
1.I Preparation of 5-nitro-2-thenoic acid through oxidation by means of hypohalides formed in the reaction medium
I. Oxidation in the presence of sodium acetate/acetic acid Into a 250-ml flask equipped with a central stirrer, a thermometer and a condenser, and containing 15.7 g (0.1 mol) of 5-nitro-2-formyl-thiophene, were introduced 16.4 g (0.2 mol) of sodium acetate, 16 ml of acetic acid and 100 ml of distilled water. The suspension was maintained under vigorous stirring and heated to about 40° C. Through a dropping-funnel, 16 g (0.1 mol) of bromine were then added in 15 to 20 minutes. At the end of this period, the temperature was 70° C. The mixture was allowed to react for one hour at 80° C., was poured into iced water containing hydrochloric acid and then extracted with ether. During the oxidation reaction, the pH of the reaction medium remained at 4.6. The ethereal phase was dried on sodium sulphate and evaporated to dryness by means of a rotatory evaporator. The crude product was recrystallized from a 70/30 heptane/1,2-dichloro-ethane mixture and then brought to 0° C. In this manner, 17 g of crude 5-nitro-2-thenoic acid were obtained, which represents a yield of 98.5% in crude product. Yield in pure product: 14 g or 81%. M.P. of the pure product: 161° C.
References:
US4220793,1980,A
527-72-0
505 suppliers
$5.00/10g

6317-37-9
198 suppliers
$5.00/1g
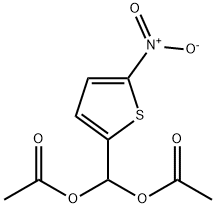
14289-24-8
9 suppliers
inquiry

6317-37-9
198 suppliers
$5.00/1g
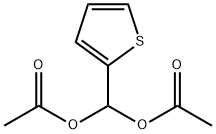
63011-97-2
16 suppliers
inquiry

6317-37-9
198 suppliers
$5.00/1g