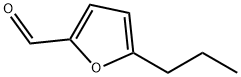
5-PROPYL-FURAN-2-CARBALDEHYDE synthesis
- Product Name:5-PROPYL-FURAN-2-CARBALDEHYDE
- CAS Number:14497-27-9
- Molecular formula:C8H10O2
- Molecular Weight:138.16
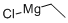
2386-64-3
215 suppliers
$45.00/5ml
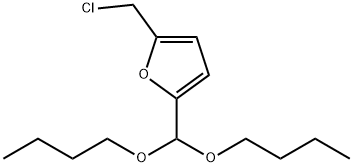
126080-78-2
0 suppliers
inquiry

14497-27-9
28 suppliers
$182.00/500mg
Yield:14497-27-9 83%
Reaction Conditions:
with bis(acetylacetonate)nickel(II);Allyl ether in tetrahydrofuran at -30; for 1 h;Inert atmosphere;
Steps:
1 Example 1. 5-Propylfuran-2-carbaldehvde
Example 1. 5-Propylfuran-2-carbaldehvde [ΘΘ85] A flask was charged with CMFDBA 6 (1.013 g, 3.687 mmol), Ni(acac)2 (56 mg, 0,22 mmol) and a stir bar. The vessel was evacuated and backfilled with argon, after which dry TFfF (20 mL) and diallyl ether (0.45 mL, 0.36 g, 3.7 mmol) were added. The mixture was stirred for 5 min and then cooled to -30 °C. Ethyl magnesium chloride (2M in THF, 3.70 mL, 7.40 mmol) was added dropwise and the resulting yellow solution was stirred at -30 °C for 1 h. IN HCl (100 mL) was added and the mixture was allowed to come to rt and stirred for 1 h. Dichloromethane (20 rnL) was added, the mixture was agitated, and the organic layer was separated. The aqueous layer was extracted with dichloromethane (2 30 rnL) and the combined organic layer was dried over Na2SC>4. The solvent was evaporated and the residue was chromatographed on silica gel (15: 1 hexanes/ethyl acetate) to give 7 as a yellow oil (0.421 g, 83%). 1H NMR (CDCI3, 300 MHz): δ 9.51 (s, 1H), 7.16 (d, ./ 4 5 Hz, i l l). 6.23 (d, ./ 4 5 Hz, l i s). 2.69 (t, ./ 7.5 Hz, 2H), 1.72 (m, 2H), 0.97 (. ./ 7.5 Hz, 3H), 5 C NMR (CDCI3, 75 MHz): δ 176,9, 163.9, 151 .8, 123.5, 108,7, 30.3, 20,9, 13,6.
References:
WO2017/48864,2017,A1 Location in patent:Paragraph 0085
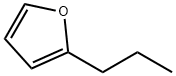
4229-91-8
67 suppliers
$15.00/1g

14497-27-9
28 suppliers
$182.00/500mg
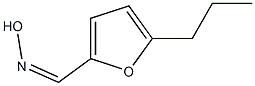
89114-37-4
0 suppliers
inquiry

14497-27-9
28 suppliers
$182.00/500mg
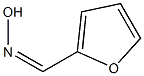
1450-58-4
6 suppliers
inquiry

14497-27-9
28 suppliers
$182.00/500mg
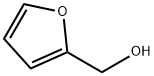
98-00-0
488 suppliers
$23.00/50g

14497-27-9
28 suppliers
$182.00/500mg