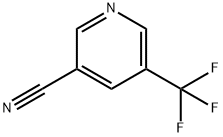
5-(TRIFLUOROMETHYL)NICOTINONITRILE synthesis
- Product Name:5-(TRIFLUOROMETHYL)NICOTINONITRILE
- CAS Number:951624-83-2
- Molecular formula:C7H3F3N2
- Molecular Weight:172.11
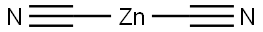
557-21-1
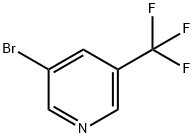
436799-33-6

951624-83-2
Example 1.1.27: Synthesis of (5-(trifluoromethyl)pyridin-3-yl)methanamine: 3-bromo-5-(trifluoromethyl)pyridine (1.0 g, 4.42 mmol, 1 eq.) was dissolved in 20 mL of anhydrous DMF. The solution was degassed by argon bubbling. Zinc cyanide (Zn(CN)2, 0.312 g, 2.65 mmol, 0.6 eq.) and tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) were subsequently added and the resulting reaction mixture was heated to 80 °C and stirred overnight. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with ether (Et2O). The organic and aqueous layers were separated by adding 28% ammonia (NH4OH) with continuous stirring. The organic layer was sequentially washed three times with water, once with brine and dried with anhydrous sodium sulfate (Na2SO4). After filtration to remove inorganic salts, the filtrate was concentrated under reduced pressure. Finally, it was purified by silica gel fast column chromatography to obtain 5-(trifluoromethyl)nicotinonitrile (0.310 g, 1.95 mmol, 44% yield).
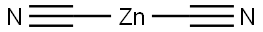
557-21-1
106 suppliers
$12.00/5g

436799-33-6
197 suppliers
$6.00/250mg

951624-83-2
54 suppliers
$58.60/250mg
Yield: 44%
Reaction Conditions:
Stage #1:dicyanozinc;3-bromo-5-(trifluoromethyl)pyridine;tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 80;
Stage #2: with ammonia in diethyl ether;water;N,N-dimethyl-formamide at 20;
Steps:
1.1.27
Example 1.1.27: (5-(trifluoromethyl)pyridin-3-yl)methanamine; 3-bromo-5-(trifluoromethyl)pyridine (1.0 g, 4.42 mmol, 1 eq) was dissolved in 20 mL anhydrous DMF. The solution was degassed by bubbling through with Ar. Zn(CN)2 (0.312 g, 2.65 mmol, 0.6 eq) and Pd(PPh3)4 were added, and the resulting solution was heated to 80 0C with stirring overnight. The reaction was cooled to room temperature and diluted with Et2O. NH4OH (28%) was added with stirring and the layers were separated. The organic layer was washed with water (x3), brine (xl), and dried over Na2SO4. The inorganics were filtered off, and the reaction mixture was concentrated in vacuo. Purification via flash chromatography on silica gel yielded 0.310 g (1.95 mmol, 44% yield) of 5- (trifluoromethyl)nicotinonitrile.
References:
COMENTIS, INC.;PURDUE RESEARCH FOUNDATION WO2009/42694, 2009, A1 Location in patent:Page/Page column 84
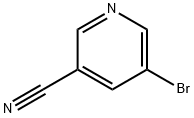
35590-37-5
237 suppliers
$6.00/250mg
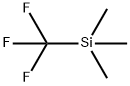
81290-20-2
417 suppliers
$13.00/1g

951624-83-2
54 suppliers
$58.60/250mg