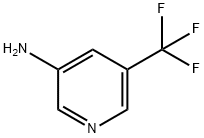
5-Trifluoromethyl-pyridin-3-ylamine synthesis
- Product Name:5-Trifluoromethyl-pyridin-3-ylamine
- CAS Number:112110-07-3
- Molecular formula:C6H5F3N2
- Molecular Weight:162.11
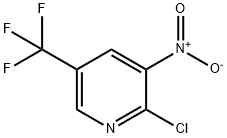
72587-15-6

112110-07-3
Step Ia: 5-(Trifluoromethyl)-2-chloro-3-nitropyridine (0.850 g, 3.75 mmol) was dissolved in methanol (17 mL), and palladium carbon (800 mg, 10% wetted, 0.375 mmol) was added. The reaction mixture was hydrogenated for 16 h at 60 psi hydrogen pressure and 20 °C. The progress of the reaction was monitored by LCMS to confirm that the starting material (M + H 213/215, 3:1) and hydroxylamine intermediate were completely consumed and a mixture of partially reduced product (M + H 163; UVmax 214, 258, 332 nm) and completely reduced product (M + H 169; no UV absorption) was detected. After completion of the reaction, the reaction mixture was filtered and the filter cake was washed well with methanol. The filtrate was concentrated in vacuum to give 0.74 g of product in the form of HCl salt. Subsequently, the product was purified by preparative HPLC/MS using a 30 mm × 100 mm C18 column; the mobile phase was 20% acetonitrile-water (containing 0.1% NH4OH), with an initial hold of 1 min and a gradient up to 40% over 6 min; the flow rate was 60 mL/min; the detector was set at m/z 163; and the product retention time was 4.4 min. Fractions containing the pure product were collected, combined and concentrated to give 52 mg of the product as an oil in 8% yield.1H NMR (CDCl3) δ 8.26 (s, 1H); 8.24 (d, 1H); 7.15 (t, 1H); 4.00 (s, 2H).
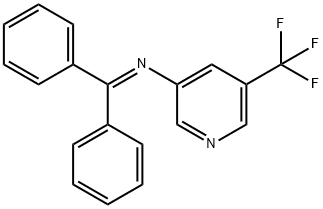
1529769-93-4
0 suppliers
inquiry

112110-07-3
150 suppliers
$19.00/250mg
Yield: 90%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;water at 20; for 2 h;
Steps:
2 Step 2: Synthesis of 5-(trifluoromethyl)pyridin-3-amine
Dissolve the imine obtained in Step 1 (850 mg, 2.6 mmol) in THF (10 mL), add water (2 mL), IN HCI solution (5 mL), stir the reaction at room temperature for 2 hrs. After the reaction, extract with EtOAc, wash the organic layer with saturated NaHC03 solution and brine sequentially, dry over anhydrous Na2S04, and concentrate under reduced pressure to give the crude product. Purification by chromatography (silica gel, EtOAc:PE=l :l) affords the target compound (380 mg, 90%).
References:
CROWN BIOSCIENCE INC. (TAICANG);ZHANG, Deyi;ZHANG, Ruihao;ZHONG, Boyu;SHIH, Chuan WO2014/418, 2014, A1 Location in patent:Page/Page column 83
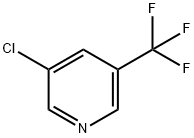
85148-26-1
211 suppliers
$32.00/1g

112110-07-3
150 suppliers
$19.00/250mg

72587-15-6
174 suppliers
$25.00/1g

112110-07-3
150 suppliers
$19.00/250mg

99368-67-9
137 suppliers
$7.00/250mg

112110-07-3
150 suppliers
$19.00/250mg
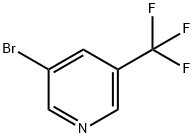
436799-33-6
197 suppliers
$6.00/250mg

112110-07-3
150 suppliers
$19.00/250mg