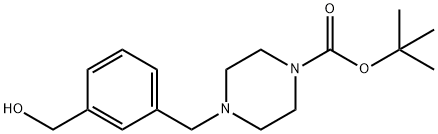
TERT-BUTYL 4-[3-(HYDROXYMETHYL)BENZYL]TETRAHYDRO-1(2H)-PYRAZINECARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-[3-(HYDROXYMETHYL)BENZYL]TETRAHYDRO-1(2H)-PYRAZINECARBOXYLATE
- CAS Number:500013-39-8
- Molecular formula:C17H26N2O3
- Molecular Weight:306.4
![3-[[4-(TERT-BUTOXYCARBONYL)PIPERAZIN-1-YL]METHYL]BENZOIC ACID](/CAS/GIF/500013-38-7.gif)
500013-38-7
40 suppliers
$82.00/250mg
![TERT-BUTYL 4-[3-(HYDROXYMETHYL)BENZYL]TETRAHYDRO-1(2H)-PYRAZINECARBOXYLATE](/CAS/GIF/500013-39-8.gif)
500013-39-8
25 suppliers
$205.00/1g
Yield:500013-39-8 23%
Reaction Conditions:
Stage #1: tert-Butyl 4-(3-carboxybenzyl)piperazine-1-carboxylatewith 4-methyl-morpholine;isobutyl chloroformate in tetrahydrofuran at 0; for 0.75 h;
Stage #2: with sodium tetrahydroborate in water at 0 - 20; for 2 h;
Steps:
18.C 18C: tert-Butyl 4-(3-(hydroxymethyl)benzyl)piperazine-1-carboxylate
Isobutyl chloroformate (0.47 ml, 3.64 mmol) was slowly added to an ice-cold solution of tert-butyl 4-(3-carboxybenzyl)piperazine-1-carboxylate (1.06 g, 3.31 mmol) and N-methylmorpholine (0.80 ml, 7.28 mmol) in THF (15 ml). The solution was stirred at 0°C for 45 min and then filtered. The filtrate was added to an ice-cold solution of sodium borohydride (313 mg, 8.27 mmol) in water (10 ml). The stirred mixture was allowed to warm to room temperature over 2 h and then concentrated in vacuo. The residue was taken up in EtOAc and the solution was washed with water and brine then concentratedin vacuo. The residue was purified by flash chromatography on silica gel (eluant EtOAc) to give a white solid identified as tert-butyl 4-(3-(hydroxymethyl)benzyl)piperazine-1-carboxylate (230 mg, 23%).
References:
EP1449844,2004,A1 Location in patent:Page 31