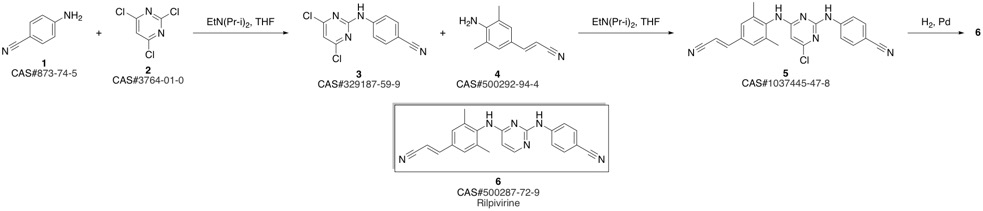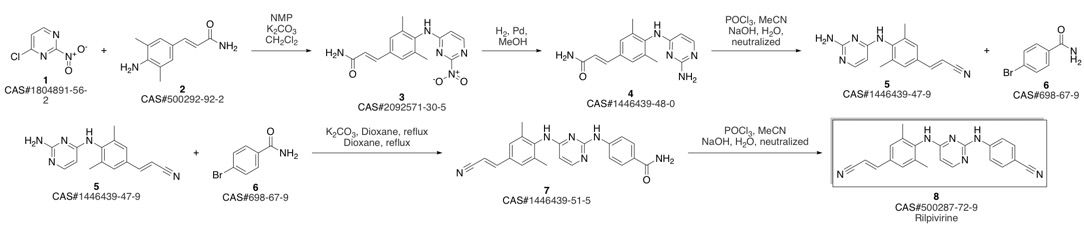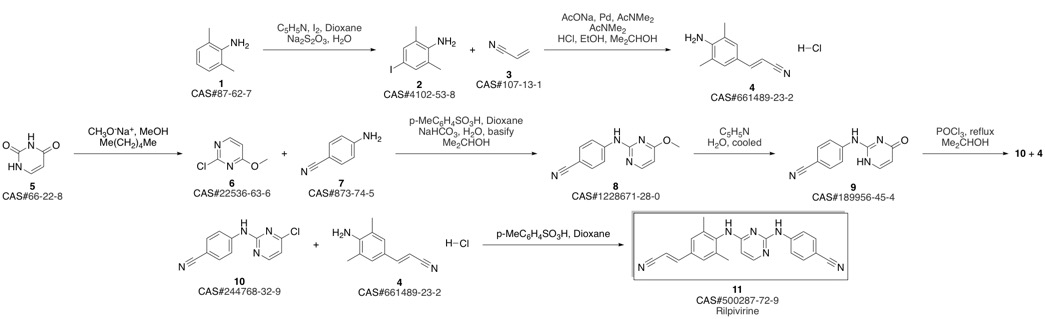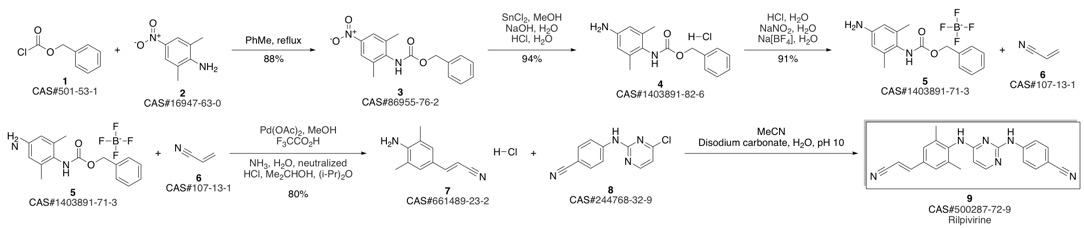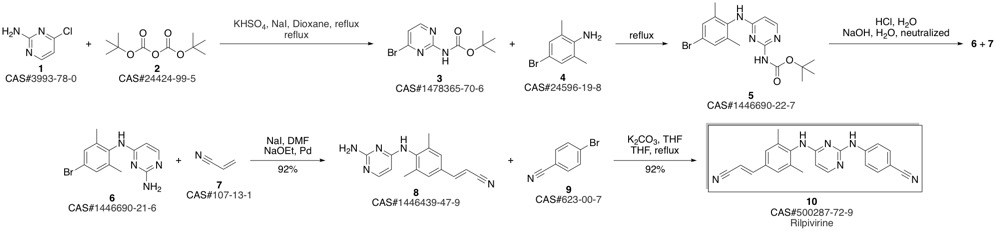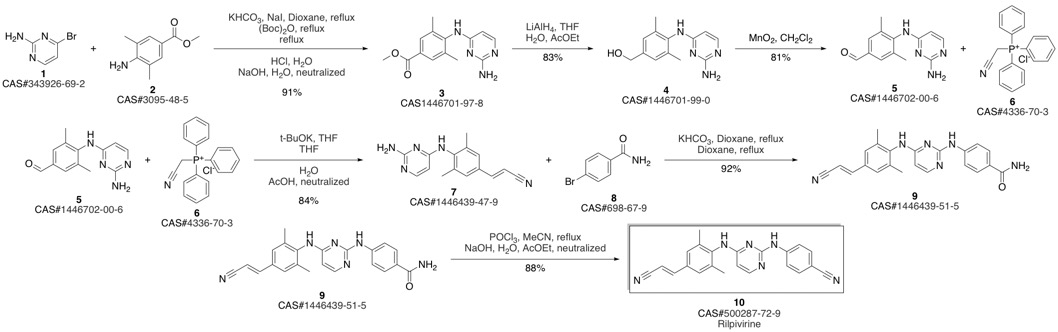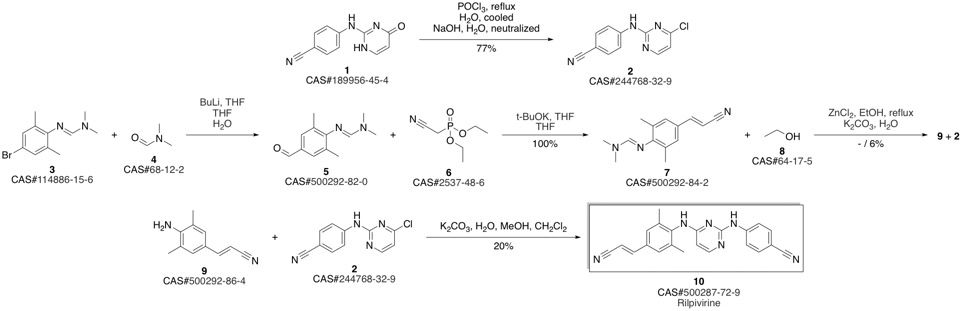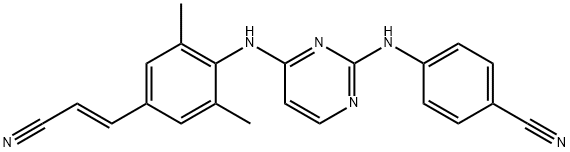
4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile synthesis
- Product Name:4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile
- CAS Number:500287-72-9
- Molecular formula:C22H18N6
- Molecular Weight:366.42
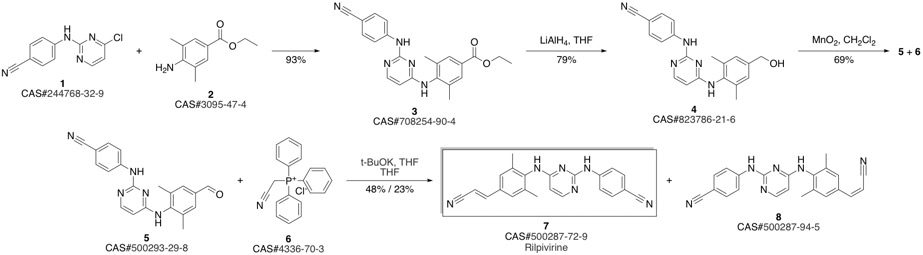
Guillemont, Jerome; Pasquier, Elisabeth; Palandjian, Patrice; Vernier, Daniel; Gaurrand, Sandrine; Lewi, Paul J.; Heeres, Jan; de Jonge, Marc R.; Koymans, Lucien M. H.; Daeyaert, Frits F. D.; Vinkers, Maarten H.; Arnold, Edward; Das, Kalyan; Pauwels, Rudi; Andries, Koen; de Bethune, Marie-Pierre; Bettens, Eva; Hertogs, Kurt; Wigerinck, Piet; Timmerman, Philip; Janssen, Paul A. J. Synthesis of novel diarylpyrimidine analogues and their antiviral activity against human immunodeficiency virus type 1. Journal of Medicinal Chemistry. Volume 48. Issue 6. Pages 2072-2079. Journal. (2005).
![3-{4-[2-(4-Cyano-phenylamino)-1-oxy-pyrimidin-4-ylamino]-3,5-dimethyl-phenyl}-acrylamide](/CAS/20180808/GIF/500288-66-4.gif)
500288-66-4
15 suppliers
inquiry
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](/CAS/GIF/500287-72-9.gif)
500287-72-9
261 suppliers
$12.00/1mg
Yield:500287-72-9 93.9%
Reaction Conditions:
with trichlorophosphate at 20;Reflux;
Steps:
3 Synthesis of compound V:
Add compound VI (10.00g, 26.0mmoL) and 80ml phosphorous oxychloride as solvent in a 500mL three-necked flask. Stir for 2-3h at 20-30, then heat to reflux for 3h. TLC tracked until the compound VI remained unchanged, the temperature was lowered to 5-10°C, the stirring was continued for 1 h, and then suction filtered and dried to obtain 9.05 g of a light yellow solid, which was the crude compound V, with a yield of 95.0%. Add all the above-mentioned light yellow solids into a 500ml three-necked flask containing 300ml of acetone, heat it to clear, and then add 0.8g of activated carbon. Incubate and stir for 1 hour. After filtering while hot, the filtrate is spin-dried to obtain 8.50 g of a white solid, which is a pure product of compound V, with a purity of 99.74% and a yield of 93.9%.
References:
CN112010810,2020,A Location in patent:Paragraph 0034; 0067-0070
1538550-43-4
3 suppliers
inquiry
873-74-5
658 suppliers
$9.00/1g
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](/CAS/GIF/500287-72-9.gif)
500287-72-9
261 suppliers
$12.00/1mg
500292-90-0
1 suppliers
inquiry
![4-[(4-CHLORO-2-PYRIMIDINYL)AMINO]BENZONITRILE](/CAS/GIF/244768-32-9.gif)
244768-32-9
177 suppliers
$69.00/1/ G
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](/CAS/GIF/500287-72-9.gif)
500287-72-9
261 suppliers
$12.00/1mg
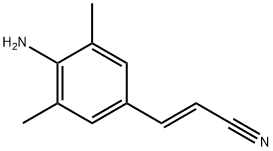
500292-94-4
42 suppliers
inquiry
![4-[(4-CHLORO-2-PYRIMIDINYL)AMINO]BENZONITRILE](/CAS/GIF/244768-32-9.gif)
244768-32-9
177 suppliers
$69.00/1/ G
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](/CAS/GIF/500287-72-9.gif)
500287-72-9
261 suppliers
$12.00/1mg
![4-[(4-CHLORO-2-PYRIMIDINYL)AMINO]BENZONITRILE](/CAS/GIF/244768-32-9.gif)
244768-32-9
177 suppliers
$69.00/1/ G
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](/CAS/GIF/500287-72-9.gif)
500287-72-9
261 suppliers
$12.00/1mg