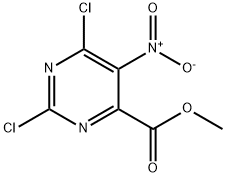
Methyl 2,6-dichloro-5-nitropyriMidine-4-carboxylate synthesis
- Product Name:Methyl 2,6-dichloro-5-nitropyriMidine-4-carboxylate
- CAS Number:52047-13-9
- Molecular formula:C6H3Cl2N3O4
- Molecular Weight:252.01
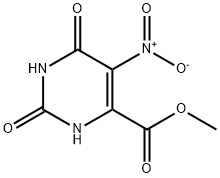
6311-73-5

52047-13-9
B. Preparation of methyl 2,6-dichloro-5-nitropyrimidine-4-carboxylate (Compound 3). Methyl 5-nitro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidine-4-carboxylate (Compound 2, 1.00 g, 3.97 mmol) was dissolved in phosphorus trichloride (4 mL). N,N-diethylaniline (1.3 mL) was added slowly and dropwise at room temperature. The reaction mixture was stirred at room temperature for 30 minutes, followed by heating and refluxing for 20 minutes. Upon completion of the reaction, the excess trichlorophosphorus was removed by concentration under reduced pressure. The concentrated oily residue was carefully poured into an ice-water mixture (10 mL) to quench the reaction. The aqueous phase was extracted with ethyl acetate (20 mL x 2) and the organic phases were combined. The organic phase was washed sequentially with 0.5 M hydrochloric acid (10 mL), saturated sodium bicarbonate solution (10 mL) and saturated saline (10 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the yellow oily target product methyl 2,6-dichloro-5-nitropyrimidine-4-carboxylate (0.78 g, 78% yield). Mass spectrum (electrospray ionization) m/e 252 ([C6H3Cl2N3O4 + H]+).

6311-73-5
52 suppliers
inquiry

52047-13-9
28 suppliers
inquiry
Yield: 95%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate at 120; for 10 h;
Steps:
2 Methyl 2,6-dichloro-5-nitropyrimidine-4-carboxylate
To a mixture of methyl 2,6-dihydroxy-5-nitropyrimidine-4-carboxylate (15 g, 70 mmol) in POCI3 (50 mL), DIEA (5 mL) was added and the resulting mixture was stirred at 120 °C for 10 h. The mixture was concentrated in vacuo. The residue was partitioned between water and DCM. The organic layer was dried over anhydrous Na2S04, filtered and concentrated in vacuo to afford the desired product (17 g, 95% yield).
References:
ARAXES PHARMA LLC;LI, Liansheng;FENG, Jun;LONG, Yun, Oliver;LIU, Yuan;REN, Pingda;LIU, Yi WO2018/64510, 2018, A1 Location in patent:Page/Page column 81-82

6311-73-5
52 suppliers
inquiry

52047-13-9
28 suppliers
inquiry
17687-24-0
58 suppliers
inquiry

52047-13-9
28 suppliers
inquiry
65-86-1
448 suppliers
$5.00/100mg

52047-13-9
28 suppliers
inquiry