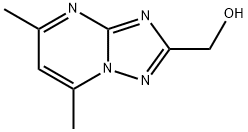
(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol synthesis
- Product Name:(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol
- CAS Number:54535-00-1
- Molecular formula:C8H10N4O
- Molecular Weight:178.19
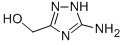
63870-39-3
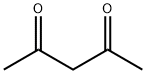
123-54-6
![(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol](/CAS/GIF/54535-00-1.gif)
54535-00-1
The general procedure for the synthesis of (5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol from (5-amino-1H-1,2,4-triazol-3-yl)methanol ethanolate and acetylacetone is as follows: refer to the literature method (Lippman, E.; Becker, V., Z. CHEM., 1974, 14, 405 ) with slight modifications: (5-amino-1H-1,2,4-triazol-3-yl)methanol ethanolate (30.7 g, 0.161 mol, from step 1 above) and 2,4-pentanedione (32.3 g, 0.323 mol, 2 eq.) were dissolved in a solvent mixture of ethanol (750 mL) and acetic acid (250 mL) and the reaction was carried out at reflux for 20 hours. At the beginning of the reaction, the mixture was a clarified solution, which gradually turned yellow as the reaction progressed. After completion of the reaction, the solvent was removed by distillation under reduced pressure to obtain a yellow paste. Ethanol (100 mL) was added to the paste, ground and stirred for 15 minutes. The paste was cooled to 5 °C (ice bath), stirring was continued for 30 min, filtered and washed with cold (0-5 °C) ethanol. The product was dried under vacuum at 25-30 °C to give 24 g (83.7% yield). The product was confirmed by 1H NMR (300 MHz, DMSO-D6): δ 2.57 (3H, s), 2.71 (3H, s), 4.63 (2H, s), 5.5 (1H, br s, OH), 7.13 (1H, s). analysis by LC-MS (APCI): the calculated value of C8H10N4O (M+H+) was 178.19; measured value (M+H+) was 179.1 m/z.
![(5-Amino-1H-[1,2,4]triazol-3-yl)-methanol](/CAS2/GIF/27277-03-8.gif)
27277-03-8
40 suppliers
$59.00/100mg

123-54-6
585 suppliers
$10.00/25ml
![(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol](/CAS/GIF/54535-00-1.gif)
54535-00-1
43 suppliers
$10.00/250mg
Yield:54535-00-1 95%
Reaction Conditions:
in acetic acid at 20; for 4 h;Heating / reflux;
Steps:
B.74.2 Step 2: (5, 7-Dimethyl- [1, 2, 4] TRIAZOLO [1, 5-A] PYRIMIDIN-2-YL)-METHANOL
To a slurry of (5-AMINO-1H- [1, 2,4] triazol-3-yl)-methanol (9.5 g, 50 mmol) from Step 1 above in acetic acid (200 mL) was added 2,4-pentanedione (5.13 mL, 50 mmol). The mixture was heated to reflux for 4 hours, and then cooled to room temperature. The product was isolated by removing the solvent by rotary evaporation (8.5 g, 95% yield). MS (ESI) : 179 (M+H).
References:
WO2004/74270,2004,A2 Location in patent:Page 327-328

63870-39-3
40 suppliers
$65.00/100mg

123-54-6
585 suppliers
$10.00/25ml
![(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol](/CAS/GIF/54535-00-1.gif)
54535-00-1
43 suppliers
$10.00/250mg
![[1,2,4]Triazolo[1,5-a]pyriMidine-2-carboxaldehyde, 5,7-diMethyl-](/CAS/GIF/55293-96-4.gif)
55293-96-4
25 suppliers
$45.00/10mg
![(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol](/CAS/GIF/54535-00-1.gif)
54535-00-1
43 suppliers
$10.00/250mg
![5,7-DIMETHYL-[1,2,4]TRIAZOLO[1,5-A]PYRIMIDINE-2-CARBOXYLIC ACID](/CAS/GIF/87253-62-1.gif)
87253-62-1
50 suppliers
$35.00/200μmol
![(5,7-dimethyl-3H-8lambda~5~-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methanol](/CAS/GIF/54535-00-1.gif)
54535-00-1
43 suppliers
$10.00/250mg