3,6-DIMETHYL-7 H-[1,2,4]TRIAZOLO[3,4-B ][1,3,4]THIADIAZINE synthesis
- Product Name:3,6-DIMETHYL-7 H-[1,2,4]TRIAZOLO[3,4-B ][1,3,4]THIADIAZINE
- CAS Number:552881-02-4
- Molecular formula:C6H8N4S
- Molecular Weight:168.22
Yield:552881-02-4 53%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20; for 20 h;
Steps:
15
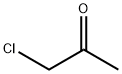
Mixture of hiocarbohydrzed (100 parts), acetic acid (200 parts) was allowed to react under refluxing by heating for 30 minutes. The mixture was cooled down to 20°C, and deposited crystal was collected by filtration. The crystal was washed by water, and dried, to thereby obtain 98 parts of intermediate (II-1c) (yield=78%). Mixture of the intermediate (II-1c) (98 parts), potassium carbonate (207 parts), and acetonitrile (500 parts) was added chloroacetone (60 parts) at room temperature, and stirred for 20 hours. A deposited crystal was collected by filtration. The crystal was washed by water, and dried, to thereby obtain 68 parts of intermediate (Il-lb) (yield=53%) . Mixture of the intermediate (II-1b) (98 parts), and acetic anhydride (240 parts) was allowed to react under refluxing by heating for 3 hours. The reaction liquid was cooled down to room temperature. A deposited crystal was washed by water, and dried, to thereby obtain mixture of intermediate (II-a). Mixture liquid of mixture of intermediate (II-1a) (10 parts), triethyl orthoformate (3.5 parts) and acetic acid (50 parts) was stirred at 80°C for 3 hours. The reaction liquid was then cooled down to room temperature, and methanol (200 mL) was dropped therein . A deposited crystal was collected by filtration, washed by water and methanol, to thereby obtain 5 parts of Compound (II-1). M/Z=282
References:
WO2013/146332,2013,A1 Location in patent:Paragraph 0218
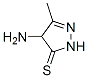
20939-15-5
67 suppliers
$75.00/100mg
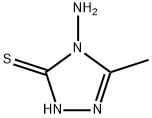
78-95-5
0 suppliers
$21.50/5G
![3,6-DIMETHYL-7 H-[1,2,4]TRIAZOLO[3,4-B ][1,3,4]THIADIAZINE](/StructureFile/ChemBookStructure6/GIF/CB0270766.gif)
552881-02-4
14 suppliers
$583.00/1g

20939-15-5
67 suppliers
$75.00/100mg
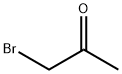
598-31-2
120 suppliers
$60.00/50mg
![3,6-DIMETHYL-7 H-[1,2,4]TRIAZOLO[3,4-B ][1,3,4]THIADIAZINE](/StructureFile/ChemBookStructure6/GIF/CB0270766.gif)
552881-02-4
14 suppliers
$583.00/1g

78-95-5
0 suppliers
$21.50/5G

20939-15-5
67 suppliers
$75.00/100mg
![3,6-DIMETHYL-7 H-[1,2,4]TRIAZOLO[3,4-B ][1,3,4]THIADIAZINE](/StructureFile/ChemBookStructure6/GIF/CB0270766.gif)
552881-02-4
14 suppliers
$583.00/1g