
5-Amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile synthesis
- Product Name:5-Amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile
- CAS Number:553672-05-2
- Molecular formula:C11H16N4
- Molecular Weight:204.27
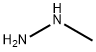
60-34-4
0 suppliers
$65.59/25g
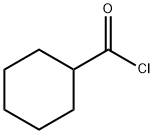
2719-27-9
356 suppliers
$16.00/25mL

109-77-3
453 suppliers
$10.00/5g

553672-05-2
25 suppliers
inquiry
Yield:553672-05-2 60%
Reaction Conditions:
Stage #1: cyclohexanylcarbonyl chloride;malononitrilewith sodium hydride in tetrahydrofuran at 0 - 20; for 1.5 h;
Stage #2: with dimethyl sulfate in tetrahydrofuran; for 3 h;Heating / reflux;
Stage #3: methylhydrazinewith triethylamine in tetrahydrofuran at 0; for 1 h;Heating / reflux;
Steps:
31.2 Production Example 31-2; 5-Amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile
Production Example 31-2 5-Amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile In a stream of nitrogen, 20.8 g (abt. 60% oil suspension 520 mmol) of sodium hydride was slowly added at 0°C to a 260 ml tetrahydrofuran solution of 17.2 g (260 mmol) of malononitrile.. Then, 35 ml (260 mmol) of cyclohexanecarbonyl chloride was added dropwise at the same temperature.. After the dropwise addition, the reactant mixture was brought to room temperature, and stirred for 1.5 hours.. Then, 30 ml (312 mmol) of dimethylsulfuric acid was added to the reaction mixture, and the mixture was heated under reflux for 3 hours.. Then, 17.4 ml (125 mmol) of triethylamine and 13.8 ml (260 mmol) of methylhydrazine were added with ice cooling, and the mixture was heated under reflux for 1 hour.. The reaction mixture was brought to room temperature, and distilled under reduced pressure.. Then, water was added to the residue, and the mixture was extracted with ethyl acetate.. The organic layer was washed with water and a saturated aqueous solution of sodium chloride, and dried over sodium sulfate.. Then, the solvent was distilled off under reduced pressure.. The residue was purified by silica gel column chromatography (chloroform/methanol = 30/1~20/1) to obtain crude crystals.. The crude crystals were further purified by recrystallization (hexane-ethyl acetate) to obtain 20.7 g (39%) of the captioned compound.. Also, the mother liquor was purified by silica gel column chromatography (hexane/ethyl acetate = 2/1) to obtain 11.3 g (21%) of the captioned compound.
References:
EP1454897,2004,A1 Location in patent:Page 24
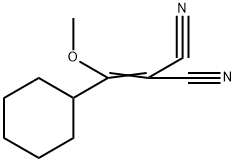
553672-04-1
1 suppliers
inquiry

553672-05-2
25 suppliers
inquiry