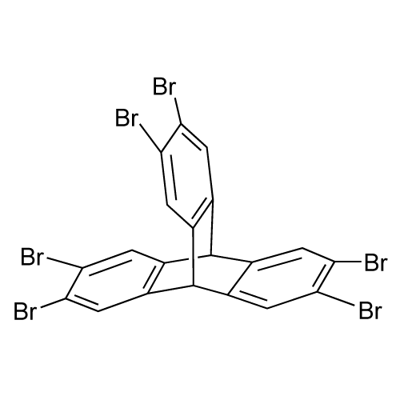
2,3,6,7,14,15-Hexabromo-9,10-dihydro-9,10-[1,2]benzenoanthracene synthesis
- Product Name:2,3,6,7,14,15-Hexabromo-9,10-dihydro-9,10-[1,2]benzenoanthracene
- CAS Number:55805-81-7
- Molecular formula:C20H8Br6
- Molecular Weight:727.71
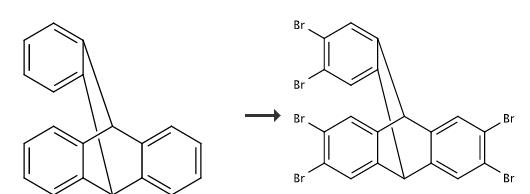
Triptycene (1.06 g, 4.18 mmol) was dissolved in chloroform (80 mL) in a round-bottom flask. Iron filings (30 mg) were added, and the solution was stirred at 25 °C. Bromine (1.35 mL, 26.3 mmol) was added, and the solution was refluxed for 1 h, during which time the initially reddish-brown solution turned reddish-orange. The flask was removed from heat, and chloroform and excess bromine were removed under vacuum. The resulting brown powder was dissolved in chloroform (100 mL) and flushed through a pad of silica using additional chloroform as eluent (100 mL). The filtrate was evaporated to dryness. The crude white powder (2.83 g, 98%) was crystallized from acetone yielding C20H8Br6 · (acetone)2 (0.88 g, 29%), mp >350 °C. The mother liquor was evaporated and the residue was crystallized from acetone to afford a second crop of crystals (0.97 g, C20H8Br6 · (acetone)2, 32%). The combined yield was 1.85 g, 61%: 2-H yield 2.83 g, 98%
477-75-8
146 suppliers
$9.00/250mg
![2,3,6,7,14,15-Hexabromo-9,10-dihydro-9,10-[1,2]benzenoanthracene](/CAS/20210111/GIF/55805-81-7.gif)
55805-81-7
46 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with bromine;iodine;iron in chloroform at 79;
Steps:
4 Synthesis of compound 4
Add triptycene (1g, 3.9mmol), liquid bromine (1.49mL, 29mmoL), iron powder (73.7mg), iodine (144.9mg), 80mL chloroform, reflux at 79°C, and check the reaction progress by nuclear magnetic After the reaction is completed, it is cooled to room temperature, filtered, and the solvent is removed in vacuo to obtain hexabromotriptycene 4 (2.5 g) as a white solid with a yield of 90%.
References:
CN112299982, 2021, A Location in patent:Paragraph 0094-0098