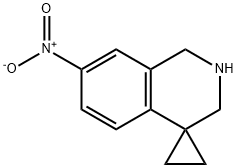
7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline] synthesis
- Product Name:7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]
- CAS Number:561297-87-8
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
![Spiro[cyclopropane-1,4'(3'H)-isoquinolin]-3'-one, 1',2'-dihydro-7'-nitro-](/CAS/20210305/GIF/1092794-09-6.gif)
1092794-09-6
1 suppliers
inquiry
![7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]](/CAS/GIF/561297-87-8.gif)
561297-87-8
13 suppliers
inquiry
Yield:561297-87-8 91.3%
Reaction Conditions:
with sodium tetrahydroborate;boron trifluoride diethyl etherate in tetrahydrofuran at 70; for 2 h;
Steps:
9.5 Fifth step: Preparation of 7′-nitro-2 ’, 3’-dihydro-1’H-spiro [cyclopropane-1,4’-isoquinoline] (Int-9-f)
Boron trifluoride diethyl ether (260.30mg, 1.83mmol) was added sodium borohydride (52.24mg, 1.37mmol) in tetrahydrofuran suspensionAfter stirring for 1 hour, a solution of compound (Int-9-e) (100 mg, 0.46 mmol) in tetrahydrofuran (8 mL) was added, and the mixture was refluxed at 70 ° C for 2 hours.The reaction solution was neutralized with saturated sodium bicarbonate, and concentrated. The residue was added to ethanol and 5N hydrochloric acid, and refluxed for 1 hour, and the solvent was concentrated.The reaction solution was neutralized with saturated potassium carbonate and extracted with ethyl acetate. The organic layers were combined and washed with saturated brine.It was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to give the title compound (282 mg, yield: 91.3%) in this step.
References:
CN110467615,2019,A Location in patent:Paragraph 0264-0265; 0278-0280
![Ethanone, 1-(2',3'-dihydro-7'-nitrospiro[cyclopropane-1,4'(1'H)-isoquinolin]-2'-yl)-2,2,2-trifluoro-](/CAS/20210305/GIF/618446-26-7.gif)
618446-26-7
0 suppliers
inquiry
![7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]](/CAS/GIF/561297-87-8.gif)
561297-87-8
13 suppliers
inquiry
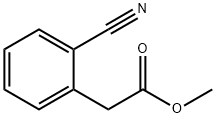
20921-96-4
90 suppliers
inquiry
![7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]](/CAS/GIF/561297-87-8.gif)
561297-87-8
13 suppliers
inquiry
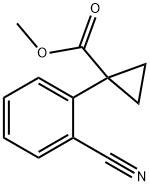
1092794-06-3
15 suppliers
inquiry
![7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]](/CAS/GIF/561297-87-8.gif)
561297-87-8
13 suppliers
inquiry
![1H-SPIRO[CYCLOPROPANE-1,4-ISOQUINOLIN]-3(2H)-ONE](/CAS/20200611/GIF/1092794-08-5.gif)
1092794-08-5
10 suppliers
inquiry
![7'-nitro-2',3'-dihydro-1'H-spiro[cyclopropane-1,4'-isoquinoline]](/CAS/GIF/561297-87-8.gif)
561297-87-8
13 suppliers
inquiry