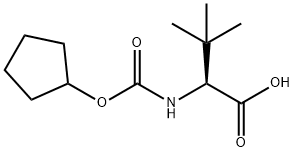
(S)-2-CYCLOPENTYLOXYCARBONYLAMINO-3,3-DIMETHYL-BUTYRIC ACID synthesis
- Product Name:(S)-2-CYCLOPENTYLOXYCARBONYLAMINO-3,3-DIMETHYL-BUTYRIC ACID
- CAS Number:572924-00-6
- Molecular formula:C12H21NO4
- Molecular Weight:243.3
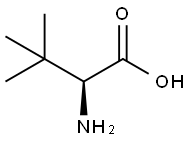
20859-02-3
620 suppliers
$9.00/5G
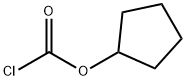
50715-28-1
165 suppliers
$14.00/1g

572924-00-6
16 suppliers
inquiry
Yield:572924-00-6 94%
Reaction Conditions:
Stage #1: L-tert-Leucinewith trimethylsilyl cyanide in acetonitrile; for 0.25 h;
Stage #2: cyclopentyl chloroformate in acetonitrile at 80;
Steps:
H.III.372.1 Example 372: Preparation of Compound 372.; Step 1:
To a solution of L-tert-leucine (2 g, 15.25 mmol) dissolved in CH3CN (50 mL) was added TMSCN (7.06 mL, 56.41 mmol) and stirred for 15 min. The reaction mixture was heated to75 C for 30 min. Cyclopentyl chloroformate (2.83 g, 19.06 mmol) was added to the reaction mixture and the reaction mixture was heated at80 C overnite, concentrated in vacuo. The residue was treated with MeOH (40 mL), stirred for 10 min, and concentrated in vacuo. The residue was adjusted pH to 8.5, and extracted with Et20(2x200mL). The aqueous layer was acidified to pH 3 and extracted withCH2CI2 (2x200mL). The combined extract was dried(MgS04), and concentrated in vacuo. The residue was recrystallized from minimal amount of Et20/hexanes to afford the product 3.48 g (94%):'H NMR (500 MHz, methanol-d4) S ppm 1.00 (s, 9 H), 1.59 (m, 2 H), 1.73 (m, 4 H), 1.84 (dd, J=5. 95,3. 20 Hz, 2 H), 3.98 (s, 1 H), 5.02(m, 1 H).
References:
WO2003/99274,2003,A1 Location in patent:Page 505-506

20859-02-3
620 suppliers
$9.00/5G
128595-07-3
160 suppliers
$10.00/1g

572924-00-6
16 suppliers
inquiry

128595-07-3
160 suppliers
$10.00/1g

572924-00-6
16 suppliers
inquiry
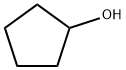
96-41-3
264 suppliers
$10.00/10g

572924-00-6
16 suppliers
inquiry