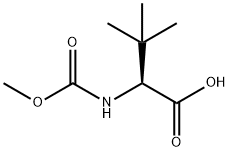
Methoxycarbonyl-L-tert-leucine synthesis
- Product Name:Methoxycarbonyl-L-tert-leucine
- CAS Number:162537-11-3
- Molecular formula:C8H15NO4
- Molecular Weight:189.21
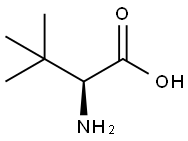
20859-02-3
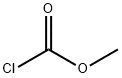
79-22-1

162537-11-3
Step 1: Synthesis of (S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid: (S)-2-amino-3,3-dimethylbutanoic acid (5.0 g, 38.6 mmol) was dissolved in a mixture of dioxane (20 ml) and 2N sodium hydroxide solution (62 ml, pH=8-9) at 0 °C. Methyl chloroformate (5.88 ml, 76.33 mmol) was added dropwise with stirring. The reaction mixture was warmed up to 60°C with continuous stirring for 18 hours. After completion of the reaction, the mixture was cooled to room temperature and extracted with dichloromethane (DCM) to separate the aqueous layer. The aqueous layer was acidified to acidity with 1N hydrochloric acid and then extracted with ethyl acetate (EtOAc). The organic layer was dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to remove the solvent. The resulting crude product was stirred and distilled in hexane to give N-methoxycarbonyl-L-tert-leucine as a white solid. Yield: 8.5 g (quantitative yield). NMR hydrogen spectrum (300MHz, CDCl3): δ5.25 (d, 1H, J=10.5Hz), 4.19 (d, 1H, J=9.6Hz), 3.70 (s, 3H), 1.03 (s, 9H). Mass spectrum: [M-1]- 188 (100%). IR spectrum (potassium bromide pressed, cm-1): 3379, 2974, 1727, 1688, 1546, 1466, 1332, 1263, 1211, 1070, 1034, 1018, 843, 696.

20859-02-3
617 suppliers
$9.00/5 g

79-22-1
0 suppliers
$38.00/1000g

162537-11-3
420 suppliers
$6.00/250mg
Yield:162537-11-3 100%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water; pH=8 - 9 at 60; for 18 h;
Steps:
8.1
Step 1: Synthesis of (S)-2-(methoxycarbonylaniino)-3,3-dimethylb tanoic acid:A stirred solution of (S)-2-amino-3,3-dimethylbutanoic acid (about 5.0 g, 38. 16 mmol) in Dioxane (about 20 ml) and sodium hydroxide (2N, 62 ml, PH = 8-9) at about 0 °C, methychlroformate (about 5.88 ml, 76.33 mmol) was added drop wise and stirred at about 60 °C for about 18 hours. The reaction mixture was cooled to room temperature, extracted with DCM, the aqueous layer was separated and acidified with I N HCl. The resulting solution was extracted with EtOAc, dried over Na2S04 and the solvent was evaporated under reduced pressure. The resulting crude was stirred in hexane and decants to afford the title compound as a solid. Wt: 8.5 g: Yield: quantitative; NMR (300 MHZ, CDCI3): δ 5.25 (d, 1 H, J = 10.5 Hz), 4. 19(d, 1 H, J = 9.6 Hz) 3.70 (s, 3H), 1 .03 (s, 9H); Mass: [M- l ]' 188 ( 100%); IR ( Br, cm 1 ): 3379, 2974, 1727, 1688, 1546, 1466, 1332, 1263. 121 1 , 1070, 1034, 1018, 843, 696.
References:
HETERO RESEARCH FOUNDATION;PARTHASARADHI REDDY, Bandi;VAMSI KRISHNA, Bandi;MANOHAR SHARMA, Vedula;RATHNAKAR REDDY, Kura;MADHANMOHAN REDDY, Musku WO2011/80562, 2011, A1 Location in patent:Page/Page column 34
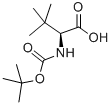
62965-35-9
430 suppliers
$6.00/5g

162537-11-3
420 suppliers
$6.00/250mg