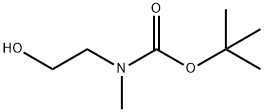
(2-HYDROXYETHYL)METHYLCARBAMIC ACID 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:(2-HYDROXYETHYL)METHYLCARBAMIC ACID 1,1-DIMETHYLETHYL ESTER
- CAS Number:57561-39-4
- Molecular formula:C8H17NO3
- Molecular Weight:175.23

109-83-1

24424-99-5

57561-39-4
General procedure: To a solution of 2-(methylamino)ethanol (500 mg, 0.53 mL, 6.66 mmol) in dichloromethane (20 mL) was added di-tert-butyl dicarbonate (1.48 g, 6.79 mmol), and the reaction was stirred at room temperature for 1 hour. After completion of the reaction, the reaction mixture was extracted with saturated aqueous sodium chloride solution and dichloromethane. The organic layers were combined, dried with anhydrous magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure to give N-BOC-N-methylaminoethanol (colorless oil, quantitative yield). The product was characterized as follows: 1H NMR (200 MHz, CDCl3) δ 3.74 (q, J = 10.5, 5.2 Hz, 2H), 3.25 (t, J = 5.2 Hz, 2H), 2.91 (s, 3H), 1.45 (s, 9H); mass spectra m/z (relative intensities) 144 (20), 102 (24), 57 (70), 44 (100).

109-83-1
460 suppliers
$10.00/25ml

24424-99-5
868 suppliers
$13.50/25G

57561-39-4
165 suppliers
$14.00/1g
Yield:57561-39-4 100%
Reaction Conditions:
in dichloromethane at 20; for 1 h;
Steps:
8.1 Step 1:
To a solution of 2-(methylamino)ethanol (500 mg, 0.53 ml, 6.66 mmol) in CH2Cl2 (20 ml) was added Boc2O (1.48 g, 6.79 mmol), followed by stirring at room temperature for 1 hour. The reaction solution was extracted with brine and CH2Cl2. The organic layer thus obtained was dried over MgSO4 and filtered. Then, the filtrate was concentrated in vacuo to obtain the object compound (colorless oil, quantitative); 1H NMR (200 MHz, CDCl3) δ 3.74 (q, J= 10.5, 5.2 Hz, 2H) 3.25 (t, J= 5.2 Hz, 2H) 2.91 (s, 3H) 1.45 (s, 9H); mass spectrum m/e (relative intensity) 144 (20) 102 (24) 57 (70) 44 (100).
References:
Korea Research Institute of Bioscience and Biotechnology;Korea Research Institute of Chemical Technology US2009/42872, 2009, A1 Location in patent:Page/Page column 19

109-83-1
460 suppliers
$10.00/25ml
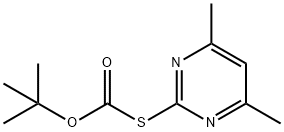
41840-28-2
127 suppliers
$20.69/5G

57561-39-4
165 suppliers
$14.00/1g

109-83-1
460 suppliers
$10.00/25ml

57561-39-4
165 suppliers
$14.00/1g
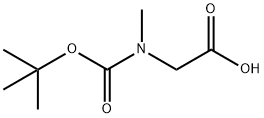
13734-36-6
307 suppliers
$11.19/5G

57561-39-4
165 suppliers
$14.00/1g

109-83-1
460 suppliers
$10.00/25ml
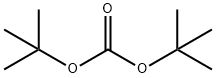
34619-03-9
35 suppliers
inquiry

57561-39-4
165 suppliers
$14.00/1g