
4,4,4-TRIFLUORO-1-(4-HYDROXY-PHENYL)-BUTANE-1,3-DIONE synthesis
- Product Name:4,4,4-TRIFLUORO-1-(4-HYDROXY-PHENYL)-BUTANE-1,3-DIONE
- CAS Number:57965-22-7
- Molecular formula:C10H7F3O3
- Molecular Weight:232.16
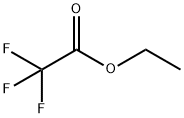
383-63-1
499 suppliers
$10.00/25g
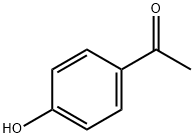
99-93-4
902 suppliers
$5.00/25g

57965-22-7
22 suppliers
$495.66/5MG
Yield:57965-22-7 66.2%
Reaction Conditions:
Stage #1: ethyl trifluoroacetate,;4-Hydroxyacetophenonewith sodium hydride in DMF (N,N-dimethyl-formamide);ethanol at 0 - 40; for 5.25 h;
Stage #2: with hydrogenchloride in DMF (N,N-dimethyl-formamide);ethanol;di-isopropyl ether;water at 0;
Steps:
9
[0639] To a solution of 4-Hydroxybenzophenone (160 g), Ethyl trifluoroacetate (182 ml), and ethanol (11 ml) in N,N-dimethylformamide (670 ml) was added portionwise sodium hydride (suspension in mineral oil, 103 g) over 15 minutes at 0 35° C. The mixture was stirring at room temperature for 2 hours, and then at 35 40° C. for 3 hours. The mixture was poured into a mixture of ice and concentrated hydrogen chloride (320 ml) (aqueous layer total 4L) and diisopropyl ether (2 L) . The aqueous layer was separated and extracted with diisopropyl ether (500 ml x 2). The combined organic layers were washed with water (500 ml x 4) and brine, dried over magnesium sulfate, and evaporated to give 415 g of solid. The solid was dissolved in diisopropyl ether (200 ml) at 65° C. The solution was added dropwise hexane (1.5 L) under stirring at room temperature. After stirring at room temperature for 1 hour, The suspension was filtered and dried under reduced pressure to give solid (first crop, 109.53 g, 40%). The mother liquid evaporated and similarly treated diisopropyl ether (20 ml) and hexane (250 ml) to give second crop (71.11 g, 26%). P0009 (first corp and second corp total, 66.2%). NMR(CDCl3); 5.65(1H, brs), 6.50(1H, s), 6.94(2H, d, J=8.8 Hz), 7.91(2H, d, J=8.8 Hz). MS(ESI+), 255.1(M+Na)+.
References:
US2004/116475,2004,A1 Location in patent:Page/Page column 26