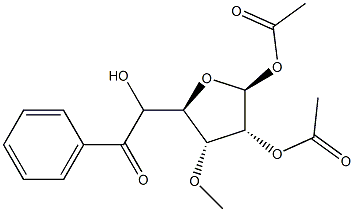
1,2-Di-O-acetyl-5-benzoyl-3-O-Methyl-beta-D-ribofuranose synthesis
- Product Name:1,2-Di-O-acetyl-5-benzoyl-3-O-Methyl-beta-D-ribofuranose
- CAS Number:58769-33-8
- Molecular formula:C17H20O8
- Molecular Weight:352.34
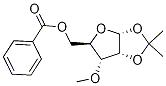
10300-20-6
12 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

58769-33-8
8 suppliers
inquiry

58800-57-0
8 suppliers
inquiry
Yield:20 % de
Reaction Conditions:
with sulfuric acid in acetic acid at 20; for 24 h;Overall yield = 88 %; Overall yield = 500 mg;
Steps:
1 2.1 5-O-Benzoyl-1,2-O-diacetyl-3-O-methyl-d-ribofuranose 9 (3)
5-O-Benzoyl-1,2-O-isopropylidene-3-O-methyl-α-d-ribofuranose (10) (500 mg, 1.62 mmol) was dissolved in HOAc (15 mL) and Ac2O (1.5 mL). Concentrated H2SO4 (0.75 mL) was added to the above mixture slowly. Then, the obtained solution was stirred at room temperature overnight and poured into ice water (100 mL). After extraction with DCM (50 mL * 3), the organic layer was washed with saturated NaHCO3 and dried with anhydrous Na2SO4. After filtration and removal of solvent in vacuo, the obtained residue was purified by flash column to give compound 3 as a mixture (α:β = 2:3, 500 mg, 88%). 1H NMR (400 MHz, CDCl3): δ 8.06 (m, 2H), 7.56 (m, 1H), 7.43 (m, 2H), 6.14 (s, 1H), 5.32 (d, 1H, J = 4.4 Hz), 4.65 (m, 1H), 4.40 (m, 1H), 4.30 (m, 1H), 4.06 (m, 1H), 3.40 (s, 3H), 2.14 (s, 3H), 1.91 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 169.7, 168.9, 166.1, 133.2, 129.8, 128.7, 128.4, 98.4, 79.7, 78.9, 73.0, 63.6, 59.3, 20.8, 20.6; ESI-MS: m/z 353.2 [M+H]+.
References:
Dou, Yan-Hui;Ding, Hai-Xin;Yang, Ru-Chun;Li, Wei;Xiao, Qiang [Chinese Chemical Letters,2013,vol. 24,# 5,p. 379 - 382]

10300-20-6
12 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

64-19-7
1601 suppliers
$10.00/25ML

58769-33-8
8 suppliers
inquiry

58800-57-0
8 suppliers
inquiry
13035-61-5
588 suppliers
$5.00/25g

58769-33-8
8 suppliers
inquiry
![(4-hydroxy-7,7-dimethyl-2,6,8-trioxabicyclo[3.3.0]oct-3-yl)methyl benz oate](/CAS/GIF/6612-91-5.gif)