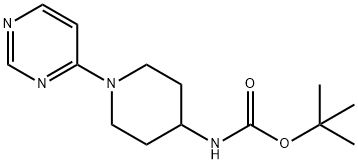
(1-PYRIMIDIN-4-YL-PIPERIDIN-4-YL)-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(1-PYRIMIDIN-4-YL-PIPERIDIN-4-YL)-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:596817-39-9
- Molecular formula:C14H22N4O2
- Molecular Weight:278.35
![[1-(2-CHLORO-PYRIMIDIN-4-YL)-PIPERIDIN-4-YL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/596817-49-1.gif)
596817-49-1
30 suppliers
$125.00/250mg

596817-39-9
11 suppliers
$496.22/5MG
Yield:596817-39-9 56%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;triethylamine in ethanol at 20 - 25; for 5 h;Inert atmosphere;
Steps:
4 Synthesis of tert-butyl (1- (pyrimidin-4-yl) piperidin-4-yl) carbamate
To a stirred solution of tert-butyl (1 -(2-chloropyrimidin-4-yl) piperidin-4-yl) carbamate (2 g, 6.39 mmol) in EtOH (300 mL) were added 10% Pd/C (300 mg) and triethylamine (646 mg, 6.39 mmol) at RT. The mixture was stirred for 5 h under H2 atmosphere (balloon pressure). After consumption of the starting material (monitored by TLC), the reaction was filtered through celite and concentrated in vacuo. The crude material was purified through silica gel column chromatography using 50% EtOAc:hexanes to afford tert-butyl (1 -(pyrimidin-4-yl) piperidin-4-yl) carbamate (1 g, 56%) as an off-white solid. ‘HNMR (DMSO-d6, 400 MHz): ? 8.45 (s, 1H), 8.14-8.12 (m, 1H), 6.85-6.80 (m, 2H), 4.28-4.25 (m, 2H), 3.54 (br s, 1H), 3.02-2.95 (m, 2H), 1.79-1.76 (m, 2H), 1.35 (s, 9H), 1.32-1.23 (m, 2H); LC-MS: 279 (M+1); (column; X-Bridge C18 (50 x 3.0 mm, 3.5 jim); RT 2.83 mm. 5 mM NH4OAc: ACN; 0.80 mL/min); TLC: 50% EtOAc:hexanes (Rf 0.2).
References:
WO2015/66696,2015,A1 Location in patent:Paragraph 0266
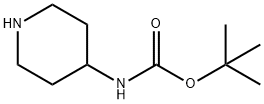
73874-95-0
442 suppliers
$10.00/250mg

596817-39-9
11 suppliers
$496.22/5MG