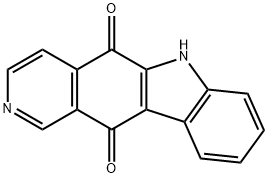
5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione synthesis
- Product Name:5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione
- CAS Number:73326-98-4
- Molecular formula:C15H8N2O2
- Molecular Weight:248.24
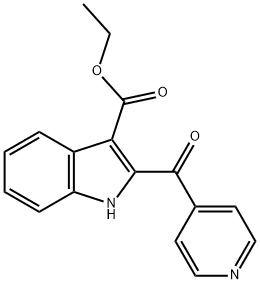
1544198-71-1
0 suppliers
inquiry
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione](/CAS/GIF/73326-98-4.gif)
73326-98-4
3 suppliers
inquiry
Yield: 68%
Reaction Conditions:
with n-butyllithium;1,1,1,3,3,3-hexamethyl-disilazane in tetrahydrofuran;hexane at -78; for 3 h;Cooling with ice;Inert atmosphere;
Steps:
Synthesis of 5H-pyrido[4,3-b]carbazole-5,11(6H)-dione (2)
A 25-mL, three-necked, round-bottomed flask is equipped with a mechanical stirrer, nitrogeninlet adapter, and fitted with a rubber septum. The flask was charged with 5 mL of dry THF and1,1,1,3,3,3-hexamethyldisilazane (0.35 mL, 1.67 mmol), and then cooled in an ice-water bathwhile 1.6M solution of n-butyllithium in hexane (0.7 mL, 1.72 mmol) was added dropwise over5-10 min. After 10 min, the resulting solution is cooled at -78°C, and a solution of 13 (0.25 g, 1mmol) in 2 mL of dry THF was added drop wise. The red reaction mixture was allowed to stirfor 3 h at -78°C. The reaction mixture was then poured into a 100 mL separatory funnelcontaining 10 mL of diethyl ether and 2 mL of 5% aqueous hydrochloric acid. The aqueous layerwas separated and extracted with 30 mL of diethyl ether. The combined organic layers werewashed with 20 mL of saturated sodium chloride solution, dried over anhydrous Na2SO4,filtered, and concentrated under reduced pressure using a rotary evaporator. The crude oilmaterial was purified by column chromatography on silica gel using hexanes/ethyl acetate (7:3)affording 2 (97 mg, 68%).S7Red solid; mp: >300 oC (lit.1 315-316 oC); IR (KBr): 3187, 1654, 1567, 1265, 1013, 810 cm-1;1H NMR (CDCl3+DMSO-d6, 500 MHz): 9.31 (s, 1H), 9.05 (d, J = 4.8 Hz, 1H), 8.27 (d, J = 8.0Hz, 1H), 8.01 (d, J = 5.2 Hz, 1H), 7.64-7.61 (m, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.36 (t, J = 8.0 Hz,1H) [Note: -NH not observed]; 13C NMR (CDCl3+DMSO-d6, 125 MHz): 184.1, 182.3, 152.4,145.0, 143.5, 141.7, 132.1, 130.9, 129.2, 129.0, 127.6, 127.1, 123.8, 123.0, 118.8; HRMS (ESI):[M+H]+ calcd for C15H8N2O2: 249.0664, found 249.0658.
References:
Ramkumar, Nagarajan;Nagarajan, Rajagopal [Tetrahedron Letters,2014,vol. 55,# 5,p. 1104 - 1106] Location in patent:supporting information
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione, 6-(methoxymethyl)-](/CAS/20210305/GIF/73540-73-5.gif)
73540-73-5
0 suppliers
inquiry
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione](/CAS/GIF/73326-98-4.gif)
73326-98-4
3 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione](/CAS/GIF/73326-98-4.gif)
73326-98-4
3 suppliers
inquiry
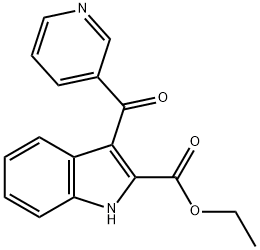
1531589-59-9
1 suppliers
inquiry
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione](/CAS/GIF/73326-98-4.gif)
73326-98-4
3 suppliers
inquiry
![5H-Pyrido[4',3':5,6]oxepino[3,2-b]indol-5-one, 6,12-dihydro-](/CAS/20210305/GIF/80757-37-5.gif)
80757-37-5
0 suppliers
inquiry
![5H-Pyrido[4,3-b]carbazole-5,11(6H)-dione](/CAS/GIF/73326-98-4.gif)
73326-98-4
3 suppliers
inquiry