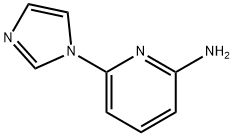
6-(1H-IMidazol-1-yl)pyridin-2-aMine synthesis
- Product Name:6-(1H-IMidazol-1-yl)pyridin-2-aMine
- CAS Number:1314355-97-9
- Molecular formula:C8H8N4
- Molecular Weight:160.18

288-32-4
1045 suppliers
$5.00/25g
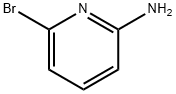
19798-81-3
446 suppliers
$5.00/1g

1314355-97-9
7 suppliers
inquiry
Yield:1314355-97-9 80%
Reaction Conditions:
with potassium carbonate in neat (no solvent) at 190; for 18 h;Schlenk technique;Inert atmosphere;
Steps:
General procedure for the synthesis of 2-imidazolylpyridines
General procedure: A broad Schlenk tube equipped with a stirring bar was loaded with 2-halopyridine (if not other noted 2-bromopyridine) (1 eq.), imidazole (3 eq.) and K2CO3 (2 eq.). The reaction mixture was degassed to 10-3 mbar and put under argon atmosphere (Argon 6.0). Thereafter, the mixture stirred at 190°C for 18h. After cooling to r.t. the mixture was diluted in water, extracted three times with chloroform and washed three times with saturated aqueous Na2CO3 solution. The combined organic phases were dried over MgSO4, filtrated and the solvent was removed under reduced pressure to leave a colorless oil or colorless solid.
References:
Raba, Andreas;Anneser, Markus R.;Jantke, Dominik;Cokoja, Mirza;Herrmann, Wolfgang A.;Kühn, Fritz E. [Tetrahedron Letters,2013,vol. 54,# 26,p. 3384 - 3387] Location in patent:supporting information