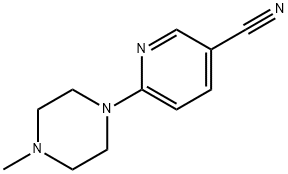
6-(4-METHYLPIPERAZIN-1-YL)NICOTINONITRILE synthesis
- Product Name:6-(4-METHYLPIPERAZIN-1-YL)NICOTINONITRILE
- CAS Number:54864-89-0
- Molecular formula:C11H14N4
- Molecular Weight:202.26
Yield:54864-89-0 85%
Reaction Conditions:
with sodium carbonate in N,N-dimethyl-formamide at 90; for 5 h;Inert atmosphere;
Steps:
15.1 Step 1
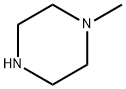
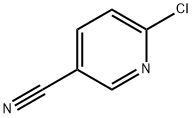
To a solution of 6-chloro-3-cyanopyridine (200 mg, 1.45 mmol) in N, N-dimethylformamide (10 mL) under nitrogen was added N-methylpiperazine (150 mg, 1.45 mmol). , Sodium carbonate (310 mg, 2.9 mmol), and the reaction system was stirred at 90 ° C for 5 hours.The reaction system was diluted with water, extracted with ethyl acetate, and the organic phase was concentrated under reduced pressure to obtain 6- (4-methylpiperazin-1-yl) nicotinonitrile (250 mg, yield: 85%) as a yellow solid.
References:
CN110467629,2019,A Location in patent:Paragraph 0213-0216