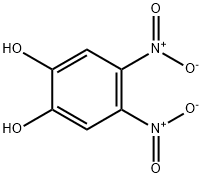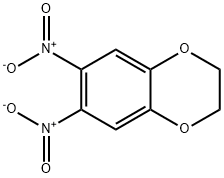
6,7-Dinitro-2,3-dihydro-benzo[1,4]dioxime synthesis
- Product Name:6,7-Dinitro-2,3-dihydro-benzo[1,4]dioxime
- CAS Number:57356-48-6
- Molecular formula:C8H6N2O6
- Molecular Weight:226.14
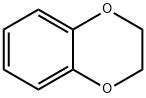
493-09-4
282 suppliers
$10.00/5G
![6,7-Dinitro-2,3-dihydro-benzo[1,4]dioxime](/CAS/GIF/57356-48-6.gif)
57356-48-6
14 suppliers
inquiry
Yield:57356-48-6 88.9%
Reaction Conditions:
Stage #1: benzo-1,4-dioxanewith HNO3;glacial acetic acid at 0 - 20; for 0.5 h;
Stage #2: with sulfuric acid;HNO3;glacial acetic acid at 20 - 80; for 2.5 h;Cooling with ice;
Steps:
1
60% concentrated nitric acid (60mL) and glacial acetic acid (30mL) are thoroughly stirred and mixed, and the ice bath is reduced to 0°C.Compound 1-1 (13 g, 95.48 mmol) was slowly added dropwise, and after the addition, the temperature was raised to room temperature and reacted for 30 min.TLC monitors the reaction. After the raw materials are reacted, the reaction solution is poured into ice water, stirred for 30 minutes and filtered with suction, the filter cake is washed with ice water, and dried to obtain a mononitrified intermediate.After drying, the intermediate was dissolved in glacial acetic acid (100 mL), and a mixed solution of fuming nitric acid (20 mL) and concentrated sulfuric acid (30 mL) cooled in an ice bath was slowly and carefully added dropwise. After the dripping is completed, warm to room temperature and react for 30 minutes, then slowly heat to 80°C, stir vigorously to react for 2 hours,The exhaust gas absorption device is used to absorb a large amount of nitrogen dioxide gas generated by the reaction. TLC monitors the reaction. After the intermediate is reacted, the reaction solution is cooled to room temperature and poured into ice water,After stirring for 30 minutes, filter with suction, wash the filter cake with ice water and dry. The dried filter cake was recrystallized by adding a mixed solution of DCM/ether (2/1, 100 mL) and heating.After cooling and crystallizing, it was filtered to obtain compound 1-2 (19.2g) as a pale yellow solid with a yield of 88.9%.
References:
CN113150005,2021,A Location in patent:Paragraph 0102-0105
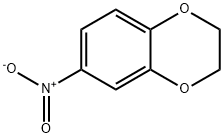
16498-20-7
137 suppliers
$19.00/1g
![6,7-Dinitro-2,3-dihydro-benzo[1,4]dioxime](/CAS/GIF/57356-48-6.gif)
57356-48-6
14 suppliers
inquiry
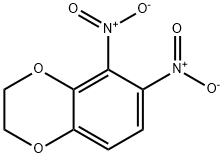
59820-94-9
3 suppliers
inquiry
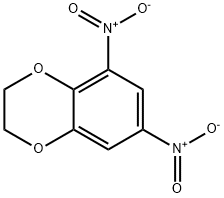
59820-95-0
0 suppliers
inquiry

16498-20-7
137 suppliers
$19.00/1g
![6,7-Dinitro-2,3-dihydro-benzo[1,4]dioxime](/CAS/GIF/57356-48-6.gif)
57356-48-6
14 suppliers
inquiry