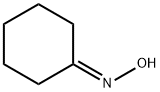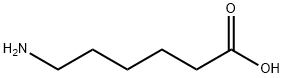
6-Aminocaproic acid synthesis
- Product Name:6-Aminocaproic acid
- CAS Number:60-32-2
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
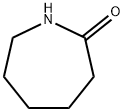
105-60-2
576 suppliers
$5.00/25g

60-32-2
609 suppliers
$5.00/10g
Yield:60-32-2 99.4%
Reaction Conditions:
with dodecatungstosilicic acid;lithium hydroxide monohydrate at 75; for 6 h;Large scale;Reagent/catalyst;
Steps:
1; 1.1; 1.2; 1.3; 1.4; 1.5; 2; 3; 4; 5; 6; 7; 8; 9; 10 Example 1. Preparation of aminocaproic acid
General procedure: 1) Hydrolysis, neutralizationWeigh 150kg of caprolactam and 600kg of purified water into a 2000L reactor and stir to dissolve.150kg of concentrated sulfuric acid was slowly added dropwise from the high-level tank with stirring, the jacket steam was heated to 75°C, and the reaction was carried out for 15 hours.After the reaction is completed, close the steam valve, open the cooling water valve, cool to about 50, add sodium hydroxide to adjust the pH of the solution to 7-8, add activated carbon to decolorize for 15 minutes, filter, wash the filter with an appropriate amount of purified water, and concentrate to remove about 450kg Water, reduce the temperature to -10 ~ -5 , filter to remove solids, the filtrate is supplemented with 300kg of water, and is referred to as filter washing solution;2) Resin adsorption and elutionOpen the resin column feed valve, pump the filtrate and washing solution obtained in step 1) into the cation resin exchange column and the anion resin exchange column in sequence, and control the flow rate to 300L/hour for adsorption. When the chemical solution flows out, collect no calcium ions and acid radicals The effluent of ions is recorded as exchange fluid;Then open the resin column purification water valve, wash the cation resin exchange column and the anion resin exchange column with purified water, combine the obtained washing liquid and exchange liquid, and record it as the solution to be crystallized, to be concentrated and crystallized.The cation resin exchange column used in this step is D113 weak acid cation resin exchange column; the anion resin column used is D301 weakly basic anion resin exchange column;The dosage ratio of the filter washing solution to the filler in the cation resin exchange column is 1L: 4kg;The dosage ratio of the filter washing solution to the filler in the anion resin exchange column is 1L: 4kg;In the adsorption step, the clarified solution is driven into an ion exchange resin to collect the exchange solution.In the washing step, the detergent is purified water, and the dosage and the feed ratio of caprolactam are 1L: 14kg.The collection standard of the exchange fluid is 2-9.3) CrystallizationAbsorb the solution to be cleaned in step 2) into a clean 1000L reaction pot, turn on the jacket to heat, heat up and concentrate to dryness, add ethanol while hot, stir to precipitate crystals, cool for more than 6 hours, filter, filter cake washed with ethanol and spin dry Wet crude aminocaproic acid was weighed.4) RefinedPut the wet crude aminocaproic acid product into a 1000L reaction pot, add 6 times the amount of mixed liquid composed of purified water and absolute ethanol in a volume ratio of 1:3, turn on the jacket and heat up to about 70 to dissolve, add activated carbon Decolorize for half an hour, filter while hot, the filtrate is concentrated to near dryness under reduced pressure, crystals are precipitated by adding 700kg of ethanol, cooled for more than 6 hours, filtered, and the crystals are washed with about 50kg of ethanol, and dried to obtain the aminocaproic acid middle product.The middle product is recrystallized according to this method, and the crystal is washed with 50 kg of ethanol. After spin-drying, the aminocaproic acid refined product is obtained, which is to be dried.5) DryLay the aminocaproic acid refined product in a stainless steel baking tray, send it to the oven, and set the temperature to 60 for drying.After drying, the finished product of aminocaproic acid is obtained, which is packaged and stored after cooling.According to the above production process, the maximum feeding scale is 300kg caprolactam, and the final yield of aminocaproic acid is 99.2% relative to caprolactam; the purity is 98.8%;
References:
CN111100025,2020,A Location in patent:Paragraph 0026-0068

4048-33-3
347 suppliers
$10.00/1g

60-32-2
609 suppliers
$5.00/10g
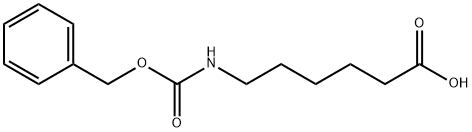
1947-00-8
164 suppliers
$8.00/5g

60-32-2
609 suppliers
$5.00/10g
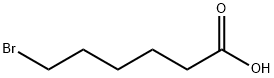
4224-70-8
355 suppliers
$7.00/5g

60-32-2
609 suppliers
$5.00/10g