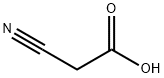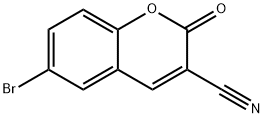
6-BROMO-3-CYANOCOUMARIN synthesis
- Product Name:6-BROMO-3-CYANOCOUMARIN
- CAS Number:76693-35-1
- Molecular formula:C10H4BrNO2
- Molecular Weight:250.05
Yield:76693-35-1 95%
Reaction Conditions:
with iodine in N,N-dimethyl-formamide; for 0.0416667 h;Microwave irradiation;Temperature;
Steps:
General procedure for the synthesis of 3-cyano-/3-cyano-4-methyl-coumarins 3a-i
General procedure: Method B (microwave conditions): A mixture of 2-hydroxybenzaldehydes/2-hydroxyacetophenones1a-i (4.71 mmol), malononitrile (2, 0.32 g, 4.71 mmol), iodine (0.03 g, 0.12mmol) and dimethylformamide (5 mL) taken in a loosely stoppered round bottom flask wassubjected to microwave irradiation for 2-5 min. Completion of the reaction was controlled bythin layer chromatography (silica gel plates using the solvent benzene: acetone, 1:1). The reaction mixture wascooled and ice-cold sodium thiosulphate solution (10 %, 30 mL) was added to remove anyiodine present in the reaction mixture. The solid that separated out was filtered, washed withwater and recrystallised from aqueous ethanol to give 3a-i.
References:
Sharma, Dinesh;Makrandi, Jagdish K. [Journal of the Serbian Chemical Society,2014,vol. 79,# 5,p. 527 - 531]
1761-61-1
388 suppliers
$5.00/5g

76693-35-1
25 suppliers
inquiry
79604-91-4
11 suppliers
$28.60/50mg

76693-35-1
25 suppliers
inquiry