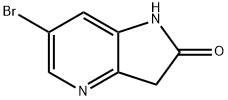
6-Bromo-4-aza-2-oxindole synthesis
- Product Name:6-Bromo-4-aza-2-oxindole
- CAS Number:1190319-62-0
- Molecular formula:C7H5BrN2O
- Molecular Weight:213.03
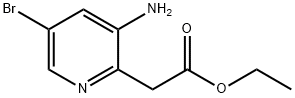
1379312-86-3

1190319-62-0
Step 3: Ethyl 2-(3-amino-5-bromopyridin-2-yl)acetate (3.5 g, 13.5 mmol) was dissolved in 97 mL of aqueous HCl (97 mmol). The reaction mixture was stirred at 50 °C for 18 hours. Upon completion of the reaction, the mixture was cooled to room temperature and solid NaHCO3 was slowly added to neutralize the acidity. Subsequently, the aqueous layer was extracted with ethyl acetate (3 x 100 mL). The organic layers were combined, washed sequentially with water and brine and dried over anhydrous Na2SO4. Concentration of the organic phase under reduced pressure afforded the target compound 6-bromo-1H-pyrrolo[3,2-b]pyridin-2(3H)-one (1.9 g, 8.12 mmol, 60% yield) as a tan solid.LCMS analysis showed a calculated value of [M + H]+ of 215 and a measured value of 215, which was in accordance with the expected isotopic distribution pattern.
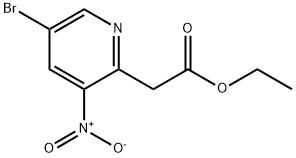
1211540-74-7
46 suppliers
inquiry

1190319-62-0
99 suppliers
inquiry
Yield: 68%
Reaction Conditions:
with iron;acetic acid at 60; for 4 h;
Steps:
6-Bromo-1 ,3 -dihydro-2H-pvrrolo [3 ,2-b Jpvridin-2-one
To a solution of Intermediate 88 (10.0 g, 34.6 mmol) in acetic acid (200 mL) wasadded iron (9 .51 g, 173 mmol). The reaction mixture was stirred at 60°C for 4 h, then10 diluted with EtOAc (200 mL ), stirred for 15 minutes, filtered through a pad of Celite, andwashed with EtOAc (3 x 200 mL). The organic layer was separated, dried overanhydrous NazS04 and concentrated in vacuo. The crude residue was dissolved in 5%MeOH in EtOAc (30 mL) and adsorbed onto Fluorosil. The resulting slurry was filteredthrough a Celite pad and washed with 5% MeOH in EtOAc (3 x 200 mL). The organic15 layer was dried over anhydrous NazS04 and concentrated in vacuo, to afford the titlecompound (5.00 g, 68%) as a brown solid. DH (400 MHz, DMSO-d6) 3.57 (s, 2H), 7.30(s, IH), 8.17 (s, IH), 10.67 (br s, IH). HPLC-MS (method 6): MH+ m/z 213.0, RT 1.31minutes.
References:
UCB BIOPHARMA SPRL;BRACE, Gareth Neil;CHAPPELL, Rose Elizabeth;DEBOVES, Hervé Jean Claude;FOLEY, Anne Marie;FOULKES, Gregory;JONES, Elizabeth Pearl;LECOMTE, Fabien Claude;QUINCEY, Joanna Rachel;SCHULZE, Monika-Sarah Elisabeth Dorothea;SELBY, Matthew Duncan;SMALLEY, Adam Peter;TAYLOR, Richard David;TOWNSEND, Robert James;ZHU, Zhaoning WO2018/229079, 2018, A1 Location in patent:Page/Page column 118

1379312-86-3
19 suppliers
inquiry

1190319-62-0
99 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1190319-62-0
99 suppliers
inquiry
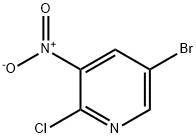
67443-38-3
377 suppliers
$5.00/1g

1190319-62-0
99 suppliers
inquiry
911434-04-3
8 suppliers
inquiry

1190319-62-0
99 suppliers
inquiry