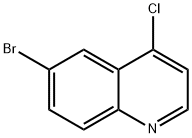
6-BROMO-4-CHLOROQUINOLINE synthesis
- Product Name:6-BROMO-4-CHLOROQUINOLINE
- CAS Number:65340-70-7
- Molecular formula:C9H5BrClN
- Molecular Weight:242.5
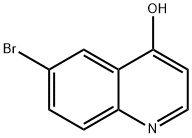
145369-94-4

65340-70-7
4.1.8 Synthesis of 6-bromo-4-chloroquinoline (11) To a 100 mL round bottom flask were sequentially added 6-bromo-4-hydroxyquinoline (10) (3.0 g, 13.45 mmol), phosphorus trichloride (20 mL) and N,N-dimethylformamide (0.5 mL). The reaction mixture was heated to reflux and stirred for 6 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was slowly poured into ice water (100 mL) and stirring was continued for 1 hour. Subsequently, the pH of the mixture was adjusted with saturated aqueous sodium bicarbonate solution to 8. The reaction mixture was extracted with ethyl acetate, the organic phases were combined and dried with anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to give the title compound 6-bromo-4-chloroquinoline (11) (2.75 g, 11.36 mmol, 84% yield) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 8.87 (d, J = 4.5 Hz, 1H, Ar-H), 8.32 (d, J = 2.0 Hz, 1H, Ar-H), 8.03 (d, J = 9.0 Hz, 1H, Ar-H), 7.99 (dd, J = 9.0, 2.0 Hz, 1H, Ar-H), 7.82 (d, J = 4.5 Hz, 1H, Ar-H). ESI-MS: m/z = 242 [M + H]+.

145369-94-4
220 suppliers
$10.00/250mg

65340-70-7
273 suppliers
$7.00/250mg
Yield:65340-70-7 98.5%
Reaction Conditions:
with trichlorophosphate for 6 h;Heating / reflux;
Steps:
1.e
The solid 6-bromo-quinolin-4-ol (52 g, 232.1 mmol) was added to phosphorus oxychloride (213.5 mL) and then the mixture was heated to reflux for 6 h to afford a light brown solution. After cooling to room temperature, the excess phosphorus oxychloride was removed under the vacuum. The remaining residue was poured into an ice-containing beaker (2 L). Then, it was slowly neutralized with solid potassium carbonate and the resulting solids were collected by filtration and washed with water. After drying in air, 55.46 g (98.5% yield) of 6-bromo-4-chloro-quinoline was isolated as a light yellow solid: EI-HRMS m/e calcd for C9H5BrClN (M+) 240.9294, found 240.9297.
References:
Chen, Li;Chen, Shaoqing;Lou, Jianping;Sidduri, Achyutharao US2006/63805, 2006, A1 Location in patent:Page/Page column 9
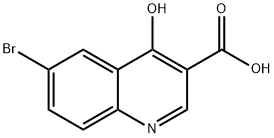
98948-95-9
124 suppliers
$10.00/250mg

65340-70-7
273 suppliers
$7.00/250mg
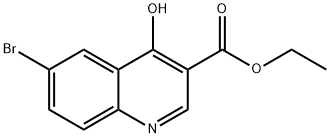
122794-99-4
148 suppliers
$7.00/250mg

65340-70-7
273 suppliers
$7.00/250mg
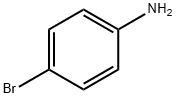
106-40-1
483 suppliers
$12.00/5g

65340-70-7
273 suppliers
$7.00/250mg
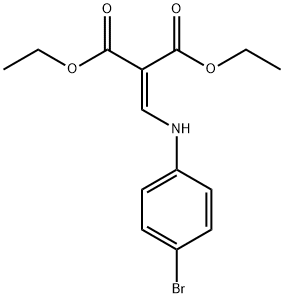
101937-44-4
42 suppliers
$120.00/50mg

65340-70-7
273 suppliers
$7.00/250mg