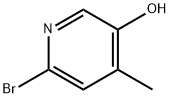
6-Bromo-4-methyl-3-pyridinol synthesis
- Product Name:6-Bromo-4-methyl-3-pyridinol
- CAS Number:1256824-49-3
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
156118-16-0
154 suppliers
$11.00/250mg

1256824-49-3
27 suppliers
inquiry
Yield:1256824-49-3 52%
Reaction Conditions:
with sulfuric acid;sodium nitrite in water at 0 - 20; for 24.5 h;
Steps:
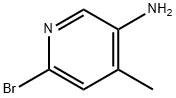
32.1 Synthesis of 6-bromo-4-methylpyridin-3-ol (32-1).
To a stirred solution of 6-bromo-4- methylpyridin-3 -amine (2.0 g, 10.7 mmol) in aqueous 40% sulfuric acid (20 mL) was added sodium nitrite (1.1 g, 16.1 mmol) at 0 °C. The resulting mixture was stirred for 30 min at 0 °C and 24 hours at ambient temperature. The resulting mixture was diluted with water (200 mL) and neutralized by potassium carbonate. The resulting mixture was extracted with ethyl acetate (3 x 100 mL). The combined organic layers was washed with brine (2 x 100 mL) and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography, eluted with 0.5-1.5% methanol in dichloromethane to afford 6-bromo-4-methylpyridin-3-ol as a light yellow solid: MS (ESI, m/z): 188.0, 190.0 [M + 1]+; 1H MR (300 MHz, OMSO-d6) δ 7.89 (s, 1H), 7.83 (s, 1H), 7.35 (s, 1H), 2.13 (s, 3H).
References:
WO2015/188368,2015,A1 Location in patent:Page/Page column 108