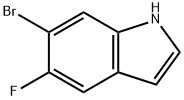
6-Bromo-5-fluoroindole synthesis
- Product Name:6-Bromo-5-fluoroindole
- CAS Number:259860-08-7
- Molecular formula:C8H5BrFN
- Molecular Weight:214.03
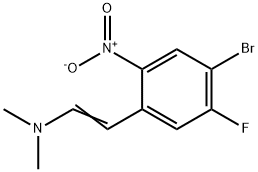
1034894-73-9

259860-08-7
General procedure for the synthesis of 5-fluoro-6-bromoindole from the compound (CAS:1034894-73-9): enamine (1.89 g, 4.38 mmol) was dissolved in ethanol (35.0 mL). To this solution, iron powder (4.42 g, 79.0 mmol) and acetic acid (35.0 mL) were added, and the reaction mixture was stirred at 90 °C overnight. After completion of the reaction, the reaction mixture was filtered and the filtrate was concentrated. The residue was purified by column chromatography (eluent: ethyl acetate/hexane, 1:9, v/v) to afford 6-bromo-5-fluoro-1H-indole as a yellow solid (0.83 g, 89% yield).1H NMR (CDCl3, 400 MHz) δ: 6.52 (m, 1H), 7.27 (m, 1H), 7.38 (d, J = 9.3 Hz, 1H) 7.57 (d, J = 5.7 Hz, 1H), 8.18 (br.s, 1H).

1034894-73-9
1 suppliers
inquiry

259860-08-7
137 suppliers
$33.00/250mg
Yield: 89%
Reaction Conditions:
with iron;acetic acid in ethanol at 90;
Steps:
The enamine (1.89 g, 4.38 mmol) was dissolved in EtOH (35.0 ml_). To this solution Fe (4.42 g, 79.0 mmol) and acetic acid (35.0 ml_) were added and the mixture was stirred at 90 °C for overnight. The resulting mixture was filtered and concentrated. The residue was purified by column chromatography (ethyl acetate-hexane; 1 :9) to afford 6- bromo-5-fluoro-1 H-indole as a yellow solid (0.83 g, 89%). 1H NMR (CDCI3, 400 MHz): 6.52 (m, 1 H), 7.27 (m, 1 H), 7.38 (d, J = 9.3 Hz, 1 H), 7.57 (d, J = 5.7 Hz, 1 H), 8.18 (br. s, 1 H).
References:
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS WO2008/77138, 2008, A1 Location in patent:Page/Page column 77
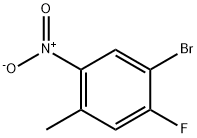
224185-19-7
205 suppliers
$7.00/1g

259860-08-7
137 suppliers
$33.00/250mg
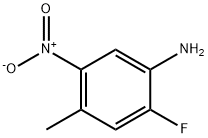
259860-00-9
52 suppliers
inquiry

259860-08-7
137 suppliers
$33.00/250mg