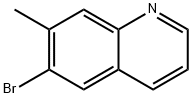
6-bromo-7-methylquinoline synthesis
- Product Name:6-bromo-7-methylquinoline
- CAS Number:122759-89-1
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
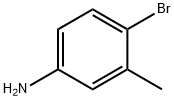
6933-10-4

56-81-5

122759-89-1
General procedure for the synthesis of 6-bromo-7-methylquinoline from 4-bromo-3-methylaniline and glycerol: [0304] A mixture of 4-bromo-3-methylaniline (20 g, 107.5 mmol), ferric sulfate (6.6 g, 43.4 mmol), glycerol (40.8 g, 440 mmol) and nitrobenzene (8.12 g, 66 mmol) was mixed with concentrated sulfuric acid (23 ml) and slowly heated. After the initial vigorous reaction subsided, the reaction mixture was refluxed for 3 h. The excess nitrobenzene was subsequently removed by evaporation. The pH was adjusted to 7-8 by adding saturated aqueous sodium bicarbonate solution to the reaction mixture and then filtered. The filtrate was extracted with dichloromethane and the organic layers were combined and dried over anhydrous sodium sulfate. The dried organic phase was filtered and concentrated in vacuum and the resulting solid was purified by fast column chromatography. The purified yellow solid was further washed with petroleum ether to afford 7.5 g of 6-bromo-7-methylquinoline in 31% yield. The structure of the product was confirmed by 1H NMR (CDCl3): δ 2.60 (s, 3H), 7.36 (m, 1H), 7.96 (s, 1H), 8.04 (m, 2H), 8.89 (m, 1H).

6933-10-4
200 suppliers
$7.00/5g

56-81-5
1771 suppliers
$5.00/25g

122759-89-1
54 suppliers
$30.00/250mg
Yield: 31%
Reaction Conditions:
Stage #1:amino-5-bromo-2-toluene;glycerol with iron(III) sulfate;sulfuric acid in nitrobenzene for 3 h;Heating / reflux;
Stage #2: with water;sodium hydrogencarbonate in nitrobenzene; pH=7 - 8
Steps:
1.4
Intermediate 4: 6-Bromo-7-methyl-quinoline; [0304] A mixture of 4-bromo-3-methylaniline (20 g, 107.5 mmol), ferric sulfate (6.6 g, 43.4 mmol), glycerol (40.8 g, 440 mmol), nitrobenzene (8.12 g, 66 mmol), and concentrated EPO
References:
SGX PHARMACEUTICALS, INC. WO2008/51808, 2008, A2 Location in patent:Page/Page column 79-80