
Glycerol synthesis
- Product Name:Glycerol
- CAS Number:56-81-5
- Molecular formula:C3H8O3
- Molecular Weight:92.09
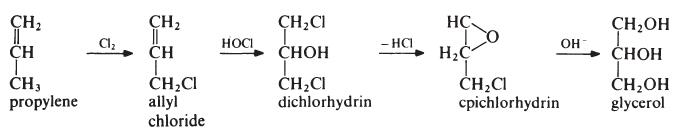
The first step is the 'hot' chlorination of propylene. A mixture of propylen(, and chlorine (4: 1 molar) is heated at about 500°C and 0.2 MPa (2 atmospheres). Under these conditions a free radical substitution reaction occurs rather than addition at the double bond and allyl chloride is the main product. This is treated with pre-formed hypochlorous acid (formed in a separate reactor by passing chlorine into water) at about 30°C to give the addition product, dichlorhydrin. The reaction mixture separates into two layers. The aqueous layer is removed to leave dichlorhydrin which is then stirred with a lime slurry to give epichlorhydrin. The epichlorhydrin is then hydrolysed to glycerol by treatment with aqueous sodium hydroxide at 150°C. The glycerol is produced as a dilute solution containing sodium chloride. Most of the water is evaporated off, during which operation the salt crystallizes out and is removed from the bottom of the evaporator to leave crude glycerol. The crude product is purified by distillation under reduced pressure. It may be noted that the intermediate epichlorhydrin has significance in its own right for the manufacture of epoxy resins.

107-18-6
0 suppliers
$24.60/100ml

56-81-5
1768 suppliers
$5.00/25g
Yield:56-81-5 100%
Reaction Conditions:
with dihydrogen peroxide;Nafion-NR50 in water at 70; for 20 h;Conversion of starting material;
Steps:
23 Example 23
Nafion-NR50 (507 mg), a 30% aqueous hydrogen peroxide solution (2.0 mL, 20.3 mmol), and allyl alcohol (0.68 mL, 9.90 mmol) were mixed and stirred at 70C for 20 hours. After the reaction, the resultant mixture was treated in the same manner as Example 1 to obtain 912 mg (9.90 mmol) of glycerol (100% yield).
References:
EP1477468,2004,A1 Location in patent:Page 7
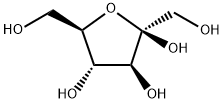
57-48-7
590 suppliers
$12.00/250G
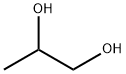
57-55-6
1657 suppliers
$5.00/1g
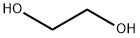
107-21-1
1374 suppliers
$10.00/25g

56-81-5
1768 suppliers
$5.00/25g
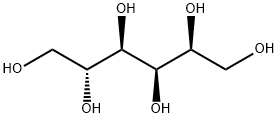
50-70-4
798 suppliers
$5.00/10g

56-81-5
1768 suppliers
$5.00/25g

67-56-1
787 suppliers
$9.00/25ml
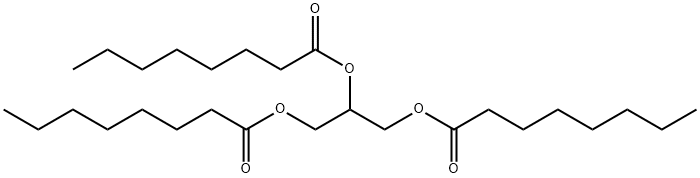
538-23-8
211 suppliers
$28.00/50mg
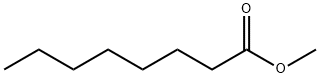
111-11-5
378 suppliers
$15.68/25ML

56-81-5
1768 suppliers
$5.00/25g
![2H-Pyran, 2-[[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methoxy]tetrahydro-](/CAS/20200611/GIF/905557-69-9.gif)
905557-69-9
0 suppliers
inquiry

56-81-5
1768 suppliers
$5.00/25g