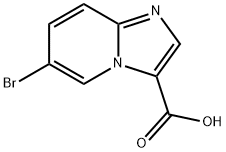
6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid synthesis
- Product Name:6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid
- CAS Number:944896-42-8
- Molecular formula:C8H5BrN2O2
- Molecular Weight:241.04
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
1. Ethyl 6-bromoimidazo[1,2-A]pyridine-3-carboxylate (12400 g, dissolved in 51 L of ethanol) was added to a stainless steel reactor of suitable capacity at room temperature and under nitrogen protection. 2. Solid compound 6 (9500 g) was added to the reaction mixture in a single addition. 3. The reaction system was heated to reflux temperature (about 78°C) with continuous stirring for 1 to 2 days. 4. The reaction process was monitored by high performance liquid chromatography (HPLC). 5. Upon completion of the reaction, the reaction mixture was cooled to room temperature. 6. Sodium hydroxide solution (9884 g NaOH solid dissolved in 38 L of water) was added slowly and dropwise over a period of 30 minutes at an internal temperature of less than 35° C. The reaction mixture was heated again for 1 to 2 days with continuous stirring. 7. The reaction mixture was again heated to reflux temperature (about 78°C) and maintained for 3 to 4 hours. 8. The reaction process was monitored by HPLC. 9. Upon completion of the reaction, the reaction mixture was cooled to a suitable temperature and solvent removal was initiated. 10. Ethanol was removed by distillation under reduced pressure at 40 to 45 °C (about 5 volumes). 11. Cool the reaction mixture to room temperature. 12. Add water (57 L; 6 vol) to the reaction mixture. 13. The aqueous phase was washed using ethyl acetate (2 x 38L) to remove organic impurities. 14. cooled the aqueous phase to 0 to 5 °C and adjusted the pH to 1-2 with concentrated hydrochloric acid (~15 L). 15. The reaction mixture was stirred at 0 to 5°C for 1 to 2 hours. 16. The mixture was filtered and the filter cake was washed sequentially with water (2 x 38 L) and acetone (2 x 19 L) and subsequently dried for 1 to 2 hours. 17. The dried solid was retransferred to a reactor of suitable capacity. 18. Heptane (95L; 10 v/v) was added and the suspension was stirred for 4 to 5 hours at room temperature. 19. Collect the solid by filtration and wash with heptane (2 x 19 L). 20. Suspend the solid (15 kg) in methanol (75 L; 5 vol) and stir at room temperature for 2 hours. 21. The suspension was filtered and the collected solid was washed with methanol (2 x 5L). 22. The solid was dried under vacuum at 50 °C to constant weight to afford 6-bromoimidazo[1,2-a]pyridine-3-carboxylic acid (Compound 8) as an off-white to white solid (10169 g, 83.3% yield; 99.2% HPLC purity; 1H NMR (DMSO-d6, 300 MHz) δ 9.4 (s, 1H), 8.3 (s, 1H), 7.85-7.67 (m, 1H). 7.85-7.67 (m, 2H)).
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g
Yield: 82%
Reaction Conditions:
with lithium hydroxide monohydrate;water in tetrahydrofuran;methanol at 60;
Steps:
1.5 Synthesis of 6-bromoimidazo[1,2-a]pyridine-3-carboxylic acid (7)
LiOH·H2O (252 mg, 6 mmol, 2 equiv) was added to the solution of the intermediate 3 (804 mg, 3 mmol, 1 equiv) in 30 mL of THF, MeOH and H2O (1:1:1). The reaction mixture was stirred at 60 °C. Upon completion of reaction as observed via TLC, the mixture was acidified with aqueous HCl (2 N) until all the solid separate out and the resulting precipitate isolated by filtration (yield 82%), obtained intermediate 7 as a white solid, 1H NMR (400 MHz, DMSO-d6) δ 13.33 (s, 1H), 9.40 (s, 1H), 8.26 (s, 1H), 7.79 (d, J = 9.5 Hz, 1H), 7.75 - 7.64 (m, 1H).
References:
Zhang, Yun;Xia, Anjie;Zhang, Shiyu;Lin, Guifeng;Liu, Jingming;Chen, Pei;Mu, Bo;Jiao, Yan;Xu, Wenwen;Chen, Mingxin;Li, Linli [Bioorganic and Medicinal Chemistry Letters,2021,vol. 41,art. no. 127881] Location in patent:supporting information
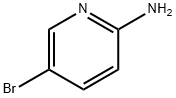
1072-97-5
702 suppliers
$9.00/1g
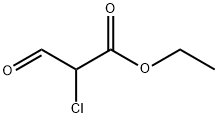
33142-21-1
130 suppliers
$12.00/250mg
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g
![6-BROMO-IMIDAZO[1,2-A]PYRIDINE-3-CARBONITRILE](/CAS/GIF/474708-98-0.gif)
474708-98-0
51 suppliers
inquiry
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g

1072-97-5
702 suppliers
$9.00/1g
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g
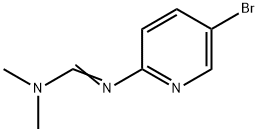
138888-98-9
17 suppliers
inquiry
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
109 suppliers
$87.00/5g