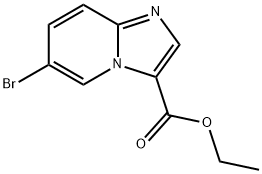
IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester synthesis
- Product Name:IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester
- CAS Number:372198-69-1
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09
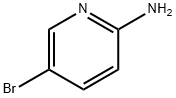
1072-97-5

1004550-24-6
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
General procedure for the synthesis of ethyl 6-bromoimidazo[1,2-A]pyridine-3-carboxylate from 2-amino-5-bromopyridine and 2-chloro-3-oxopropanoic acid ethyl ester potassium salt: In a sealed tube, 2-amino-5-bromopyridine (5.92 g, 34.2 mmol) and 2-chloro-3-oxopropanoic acid ethyl salt potassium salt (20.0 g, 106.0 mmol) were added to a solution of ethanol (140 mL) containing concentrated sulfuric acid (2.7 mL) in a solution of ethanol (140 mL). The reaction vessel was sealed by purging with nitrogen and the reaction was heated at 90 °C for 18 hours. Upon completion of the reaction, the resulting slurry was cooled to room temperature, filtered and concentrated under reduced pressure. The residue was diluted with ethyl acetate and washed sequentially with 1N sodium hydroxide solution and brine. The combined aqueous layers were back-extracted with a 95:5 solvent mixture of ethyl acetate/methanol. The combined organic extracts were dried with anhydrous sodium sulfate and filtered through a diatomaceous earth pad after decolorization with the addition of activated carbon. The filtrate was concentrated under reduced pressure and the residue was recrystallized with a 1:1 ethyl acetate/hexane solvent mixture (100 mL) to give 5.09 g (55% yield) of the target product, ethyl 6-bromoimidazo[1,2-A]pyridine-3-carboxylate, as an off-white solid. Mass spectrum (electrospray ionization) showed m/e 271.2 [M+H]+.
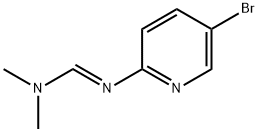
138240-34-3
2 suppliers
inquiry
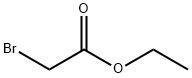
105-36-2
349 suppliers
$5.00/5 g
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg
Yield: 56%
Reaction Conditions:
with sodium hydrogencarbonate in isopropyl alcohol at 80;
Steps:
Ethyl 6-bromoimidazo [1,2-a] pyridine-3-carboxylate
Combine (E) -N '-(5-bromopyridin-2-yl) -N, N-dimethylformamidine (17.0 g, 74.53 mmol), sodium bicarbonate (12.5 g, 149.06 mmol) and Ethyl bromoacetate (37.3 g, 223.60 mmol) was added sequentially with isopropanol (200 mL). The reaction was stirred overnight at 80 degrees. LCMS showed the reaction was complete. The reaction solution was evaporated to dryness, and the residue was diluted with ethyl acetate and washed with water. The organic phase was dried over anhydrous sodium sulfate and concentrated to obtain crude product. The obtained crude product was beaten with isopropanol to obtain a pink solid compound (11.2 g, yield 56%).
References:
Shanghai Yinuo Pharmaceutical Co., Ltd.;Jiang Lei;Feng Zhiyong;Shang Ke;Shou Jianyong;Wu Danyi;Xu Yuan;Zhang Shuyun;Zhang Yi;Zhang Yuxing CN111039946, 2020, A Location in patent:Paragraph 0151-0152; 0157-0161

1072-97-5
702 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg

1072-97-5
702 suppliers
$9.00/1g
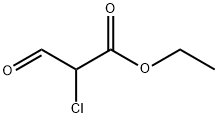
33142-21-1
130 suppliers
$12.00/250mg
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg
![6-broMo-3-iodoH-iMidazo[1,2-a]pyridine](/CAS/GIF/474706-74-6.gif)
474706-74-6
108 suppliers
$31.00/100mg
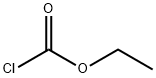
541-41-3
0 suppliers
$19.39/5g
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg

1072-97-5
702 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
114 suppliers
$14.00/250mg