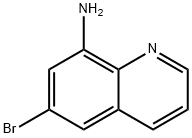
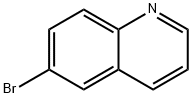
6-bromoquinolin-8-amine synthesis
- Product Name:6-bromoquinolin-8-amine
- CAS Number:57339-57-8
- Molecular formula:C9H7BrN2
- Molecular Weight:223.07
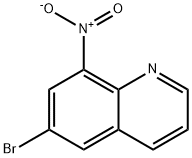
68527-67-3

57339-57-8
General procedure for the synthesis of 6-bromo-8-aminoquinoline from 6-bromo-8-nitroquinoline: to a mixed solution of 6-bromo-8-nitroquinoline (4 g, 1.58 mmol) in ethanol/acetic acid/water (50 mL/50 mL/25 mL) was added iron powder (3.18 g, 5.69 mmol). The reaction mixture was heated to reflux for 3 hours. Upon completion of the reaction, it was cooled to room temperature, neutralized with 2.5 N sodium hydroxide solution, filtered through diatomaceous earth to remove the iron powder residue, and the filter cake was washed with ethyl acetate. The filtrate was extracted with ethyl acetate (3 x 200 mL), the organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure. The oil obtained was purified by column chromatography (40% ethyl acetate/hexane) to give 3.19 g (91% yield) of yellow solid with a melting point of 142-145 °C. The residue was extracted with ethyl acetate (3 x 200 mL) and dried with anhydrous sodium sulfate.

68527-67-3
56 suppliers
inquiry

57339-57-8
53 suppliers
$184.00/250mg
Yield:57339-57-8 91%
Reaction Conditions:
with iron;acetic acid in ethanol;water; for 3 h;Heating / reflux;
Steps:
49 INTERMEDIATE 49 6-Bromo-8-amino-quinoline
To a solution of 6-bromo-8-nitro-quinoline (4 g, 1.58 mmol) in EtOH/HOAc/H2O (50 mL/50mL/25mL) was added iron metal (3.18 g, 5.69 mmol). The resulting solution was heated at reflux for 3 hours. The cooled reaction mixture was neutralized with 2.5 N NaOH, filtered through celite to remove iron solids and washed with EtOAc. The eluent was extracted into EtOAc (3 x 200 mL), combined, dried over Na2SO4 and concentrated. The resulting oil was purified by column chromatography (40% EtOAc/hexanes) affording 3.19 g (91%) of a yellow solid: mp 142-145°C.
References:
EP1147083,2004,B1 Location in patent:Page 46
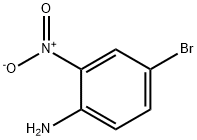
875-51-4
306 suppliers
$5.00/1g

57339-57-8
53 suppliers
$184.00/250mg
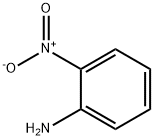
88-74-4
59 suppliers
$10.00/1g

57339-57-8
53 suppliers
$184.00/250mg
5332-25-2
400 suppliers
$6.00/5g

57339-57-8
53 suppliers
$184.00/250mg