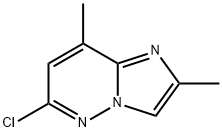
6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine synthesis
- Product Name:6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine
- CAS Number:17412-23-6
- Molecular formula:C8H8ClN3
- Molecular Weight:181.62
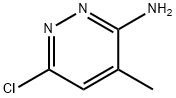
64068-00-4
120 suppliers
$40.00/250mg
598-31-2
124 suppliers
$60.00/50mg
![6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-23-6.gif)
17412-23-6
79 suppliers
inquiry
Yield:17412-23-6 54.6%
Reaction Conditions:
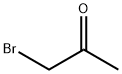
Stage #1:3-amino-6-chloro-4-methylpyridazine;1-bromoacetone in acetonitrile for 8 h;Heating / reflux;
Stage #2: with sodium hydroxide;water; pH=9
Steps:
62 Reference Example 62; Synthesis of 6-chloro-2,8-dimethylimidazo[1,2-b]pyridazine
3-Amino-6-chloro-4-methylpyridazine (5.50 g, 38.3 mmol) and bromoacetone (6.90 g, 40.0 mmol) were heated in acetonitrile (50.0 ml) for 8 hours under reflux. The reaction solution was concentrated under reduced pressure, and water (100 ml) was added to the residues which were then adjusted to pH 9 with 20% aqueous sodium hydroxide and extracted twice with ethyl acetate. The extracts were combined, dried over anhydrous magnesium sulfate and concentrated, and the residues were purified'by silica gel column chromatography (ethyl acetate : chloroform = 1 : 2), to give the title compound as white crystals. The yield was 3.80 g (54.6%). mp 109-110°C1H NMR (CDCl3, δ): 2.49-2.50(3H, m), 2.63-2.64(3H, m), 6.83-6.85(1H, m), 7.66(1H, s). IR(Nujol, cm-1): 3129, 1592, 1532, 1289, 1113, 1092, 985, 928, 843, 772.
References:
Sumitomo Chemical Takeda Agro Company, Limited EP1466527, 2004, A1 Location in patent:Page 36
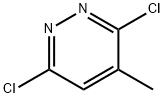
19064-64-3
272 suppliers
$6.00/5g
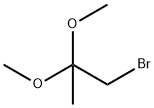
126-38-5
138 suppliers
$12.00/1g
![6-chloro-2,7-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-20-3.gif)
17412-20-3
15 suppliers
inquiry
![6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-23-6.gif)
17412-23-6
79 suppliers
inquiry

64068-00-4
120 suppliers
$40.00/250mg
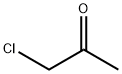
78-95-5
0 suppliers
$16.30/5 g
![6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-23-6.gif)
17412-23-6
79 suppliers
inquiry

19064-64-3
272 suppliers
$6.00/5g

598-31-2
124 suppliers
$60.00/50mg
![6-chloro-2,7-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-20-3.gif)
17412-20-3
15 suppliers
inquiry
![6-chloro-2,8-dimethyl-Imidazo[1,2-b]pyridazine](/CAS/20180703/GIF/17412-23-6.gif)
17412-23-6
79 suppliers
inquiry