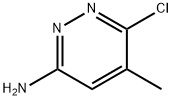
6-chloro-5-Methylpyridazin-3-aMine synthesis
- Product Name:6-chloro-5-Methylpyridazin-3-aMine
- CAS Number:66346-87-0
- Molecular formula:C5H6ClN3
- Molecular Weight:143.57
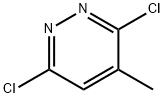
19064-64-3

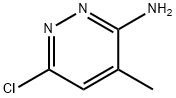
66346-87-0
64068-00-4
The general procedure for the synthesis of 3-amino-5-methyl-6-chloropyridazine and 3-amino-4-methyl-6-chloropyridazine from 3,6-dichloro-4-methylpyridazine is as follows [Linholter, S., et al., Acta Chem. Scand. 15:1660-1666 (1961)]: to a 3,6-dichloro-4-methylpyridazine (200 mg) solution in ethanol (3 mL) was added to liquid ammonia (3 mL). The reaction mixture was placed in a sealed tube and heated at 120 °C for 12 hours. After completion of the reaction, it was cooled to room temperature and the methanol and ammonia were removed by evaporation. The residue was purified by fast chromatography (eluent: ethyl acetate) to give 153 mg of a mixture of 3-amino-5-methyl-6-chloropyridazine and 3-amino-4-methyl-6-chloropyridazine (1:1 ratio) in 87% yield. The mixture could be used directly for subsequent experiments without further separation.

19064-64-3
271 suppliers
$6.00/5g

66346-87-0
127 suppliers
$9.00/100mg
Yield:66346-87-0 72.7%
Reaction Conditions:
with ammonia in ethanol;water at 100; for 48 h;
Steps:
5
EXAMPLE 5; SYNTHESIS OF IMIDAZO[1 ,2-B]PYRIDAZINE COMPOUNDS AND 7- METHYL-IMIDAZO[1 ,2-B]PYRIDAZINE COMPOUNDS [0177] Additional compounds of the invention, including illustrative compounds set forth in Table VII, were made according to the following synthetic examples. In these examples, Compound 7-12 was prepared as described above in Example 2, while compound 11 was prepared as follows:β-chloroS-methylpyridazin-S-amine 7:for 48 h.[0178] 3,6-dichloro-4-methylpyridazine 6 (1 g) was dissolved in 5 mL of ethanol and was added ammonium hydroxide (10 mL). The resulting reaction mixture was sealed in a pressure bottle and heated to 100 0C for 48 hours. The reaction mixture is cooled and the solvents were evaporated and purified by CombiFlash Companion using Hexane/DCM 40:60 solvent system (4 g normal phase RediSep Flash column with run time min at flow 18 mL/min) gave 0.640 g (72.7 %) of 7 as yellow solid).[0179] 1H-NMR (300MHz, CD3OD) 7.08 (s, 1 H), 4.72 (s, 2H), 2.27 (s, 3H), ESI-MS m/z 143.9 (M+H)+.
References:
WO2008/58126,2008,A2 Location in patent:Page/Page column 65

19064-64-3
271 suppliers
$6.00/5g

66346-87-0
127 suppliers
$9.00/100mg

64068-00-4
120 suppliers
$40.00/250mg
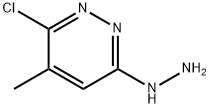
66530-56-1
13 suppliers
inquiry

66346-87-0
127 suppliers
$9.00/100mg