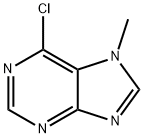
6-CHLORO-7-METHYLPURINE synthesis
- Product Name:6-CHLORO-7-METHYLPURINE
- CAS Number:5440-17-5
- Molecular formula:C6H5ClN4
- Molecular Weight:168.58
87-42-3

74-88-4

5440-17-5
To a 1L three-necked round-bottomed flask was added 6-chloro-9H-purine (15.4 g, 0.1 mol, 1 eq.) and tetrahydrofuran (155 mL) at 0 °C and protected by nitrogen in an inert atmosphere. Methylmagnesium chloride (MeMgCl, 36.6 mL, 1.0 M THF solution, 1.1 eq.) was added dropwise with stirring. The reaction mixture was continued to be stirred at 0 °C for 30 min. Subsequently, iodomethane (42.6 g, 3 eq.) was added slowly and dropwise with stirring. The resulting reaction solution was transferred to a 50 °C oil bath and the reaction was stirred for 5 hours. Upon completion of the reaction, the reaction was quenched by addition of 50 mL of aqueous ammonium chloride (NH4Cl) and extracted with dichloromethane (3 x 50 mL). The organic layers were combined and washed with brine (2 x 50 mL) and subsequently concentrated under vacuum. The crude product was recrystallized by dichloromethane/petroleum ether (1:10) to afford 6-chloro-7-methylpurine as a light green solid (7 g, 42% yield).

87-42-3
500 suppliers
$14.00/25g

74-88-4
357 suppliers
$15.00/10g

5440-17-5
72 suppliers
$57.00/100mg
Yield: 0.4 g
Reaction Conditions:
with methylmagnesium chloride in tetrahydrofuran at 70; for 12 h;
Steps:
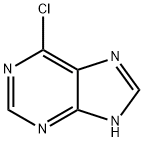
22.1 Step 1: Synthesis of 6-chloro-7-methyl-7H-purine
[0498] Procedure: To a stirred solution of 6-chloro-7H-purine (1 g, 6.469 mmol) in Dry THF (20 mL) was added MeMgCl (0.53 g, 7.086 mmol) followed by methyl iodide (1.3 g, 9.158 mmol). Reaction mixture was stirred for 12 h at 70 °C. Progress of the reaction was monitored by TLC. Reaction mixture was diluted with water (20 mL), extracted with ethyl acetate (2 x 50mL). The combined organic layer was washed with brine (10mL), dried over anhydrous sodium sulphate, filtered and evaporated under reduced pressure. The crude residue was purified by combiflash using methanol in dichloromethane to afford 6-chloro-7-methyl-7H-purine as white solid, (0.4 g, 36.46 %).1HNMR (400 MHz, DMSO-d6): δ 8.75 (s, 1H),8.71-(s, 1H), 3.96 (s, 3H). LC-MS (ES) m/z = 169.1 [M+H]+.
References:
MAVUPHARMA, INC.;GALLATIN, William Michael;ODINGO, Joshua;DIETSCH, Gregory N.;FLORIO, Vincent;VENKATESHAPPA, Chandregowda;DURAISWAMY, Athisayamani Jeyaraj WO2019/46778, 2019, A1 Location in patent:Paragraph 0498

87-42-3
500 suppliers
$14.00/25g

74-88-4
357 suppliers
$15.00/10g

5440-17-5
72 suppliers
$57.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5440-17-5
72 suppliers
$57.00/100mg

87-42-3
500 suppliers
$14.00/25g

74-88-4
357 suppliers
$15.00/10g

5440-17-5
72 suppliers
$57.00/100mg
2346-74-9
108 suppliers
$60.00/50 mg

87-42-3
500 suppliers
$14.00/25g

74-88-4
357 suppliers
$15.00/10g

5440-17-5
72 suppliers
$57.00/100mg

2346-74-9
108 suppliers
$60.00/50 mg