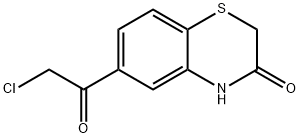
6-CHLOROACETYL-2H-1 4-BENZOTHIAZIN-3(4H& synthesis
- Product Name:6-CHLOROACETYL-2H-1 4-BENZOTHIAZIN-3(4H&
- CAS Number:145736-61-4
- Molecular formula:C10H8ClNO2S
- Molecular Weight:241.69
5325-20-2
123 suppliers
$10.00/5g

79-04-9
381 suppliers
$12.00/5g

145736-61-4
20 suppliers
$34.39/1g
Yield:145736-61-4 95.6%
Reaction Conditions:
Stage #1: chloroacetyl chloridewith aluminum (III) chloride in dichloromethane; for 0.166667 h;Cooling with ice;
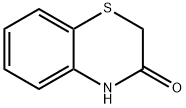
Stage #2: 2H-1,4-benzothiazin-3-one in dichloromethane at 20;Cooling with ice;
Steps:
22.1 Step 1:
Step 1: (0404) Anhydrous aluminum trichloride (1.62 g, 12.15 mmol) was added portionwise into chloroacetyl chloride (912 ul, 12.11 mmol) dissolved in methylene chloride (6 ml) under ice bath followed by stirring for 10 minutes at this temperature. Then 2H-1,4-benzothiazine-3(4H)-one 22-a (500 mg, 3.027 mmol) was added portionwise thereto, the reaction was stirred under ice bath for 4 hours and at room temperature overnight. Ice water (10 ml) and 4N hydrochloric acid (10 ml) was added sequentially. The mixture was stirred at room temperature for 2 hours. The precipitated solid was filtered, the filter cake was washed twice with ice water, dried to give 22-b as a pale yellow solid (700 mg, yield: 95.6%). 1H-NMR (300 Hz, DMSO-d6): δ ppm 10.76 (brs, 1H), 7.57 (dd, 1H), 7.50 (m, 2H), 5.11 (s, 2H), 3.54 (s, 2H).
References:
US2017/158680,2017,A1 Location in patent:Paragraph 0401; 0403; 0404