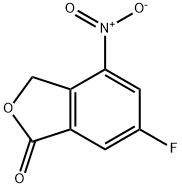
6-Fluoro-4-nitro-3H-isobenzofuran-1-one synthesis
- Product Name:6-Fluoro-4-nitro-3H-isobenzofuran-1-one
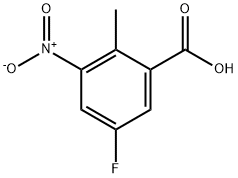
- CAS Number:1207453-90-4
- Molecular formula:C8H4FNO4
- Molecular Weight:197.12
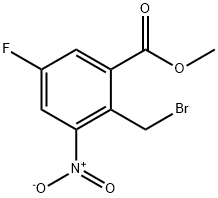
850462-65-6

1207453-90-4
The general procedure for the synthesis of 6-fluoro-4-nitroisobenzofuran-1(3H)-ones from methyl 2-(bromomethyl)-5-fluoro-3-nitrobenzoate was as follows: methyl 5-fluoro-2-methyl-3-nitrobenzoate (28 g, 130.5 mmol), N-bromosuccinimide (NBS, 27.8 g, 156.6 mmol) and benzoyl peroxide ( BPO, 3.13 g, 13.1 mmol) in carbon tetrachloride (CCl4, 400 mL) were heated to reflux and reacted overnight. The reaction was monitored by thin layer chromatography (TLC, unfolding agent ratio of petroleum ether/ethyl acetate = 15:1) to confirm complete consumption of the feedstock. Upon completion of the reaction, water (200 mL) was added and carbon tetrachloride was removed by distillation under reduced pressure. The residue was extracted with dichloromethane (DCM, 200 mL x 3). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to give the crude product methyl 2-(bromomethyl)-5-fluoro-3-nitrobenzoate (36 g, 94% yield) as a brown oil. The resulting methyl 2-(bromomethyl)-5-fluoro-3-nitrobenzoate (36 g, 123 mmol) was dissolved in 1,4-dioxane (250 mL) and water (62.5 mL) and heated to reflux for 4 days. Complete consumption of the raw material was confirmed by TLC (unfolding agent ratio petroleum ether/ethyl acetate = 15:1). Upon completion of the reaction, 1,4-dioxane was removed by distillation under reduced pressure. The residue was extracted with ethyl acetate (EtOAc, 300 mL x 4). The organic layers were combined, washed with saturated saline, dried over anhydrous sodium sulfate and concentrated to give the crude product. The crude product was purified by silica gel column chromatography (eluent tapered from petroleum ether to petroleum ether/ethyl acetate=5:1) to afford 6-fluoro-4-nitroisobenzofuran-1(3H)-one (19.2 g, yield 79%) as a white solid. The structure of the product was confirmed by 1H-NMR (400 MHz, CDCl3) and LC-MS (ESI): 1H-NMR δ (ppm): 5.74 (s, 2H), 7.97-7.98 (dd, 1H), 8.24-8.27 (dd, 1H); LC-MS (ESI) m/z: 198 (M+1)+.

850462-65-6
24 suppliers
inquiry

1207453-90-4
99 suppliers
$30.00/1g
Yield:1207453-90-4 79%
Reaction Conditions:
with water in 1,4-dioxane; for 96 h;Reflux;
Steps:
63B
A mixture of methyl 5-fluoro-2-methyl-3-nitrobenzoate (28 g, 130.5 mmol), NBS (27.8 g, 156.6 mmol) and BPO (3.13 g, 13.1 mmol) in CCl4 (400 mL) was heated to reflux overnight. TLC (petroleum ether/EtOAc=15:1) showed the starting material was consumed completely. Water (200 mL) was added and CCl4 was removed under reduced pressure. The residue was extracted with DCM (200 mL×3). The combined organic layers were washed with brine, dried over Na2SO4, concentrated to give crude methyl 2-(bromomethyl)-5-fluoro-2-methyl-3-nitrobenzoate (36 g, yield 94%) as a brown oil. A mixture of methyl 2-(bromomethyl)-5-fluoro-2-methyl-3-nitrobenzoate (36 g, 123 mmol) in 1,4-dioxane (250 mL) and water (62.5 mL) was heated to reflux for 4 days. TLC (petroleum ether/EtOAc=15:1) showed the starting material was consumed completely. Dioxane was removed under reduced pressure. The residue was extracted with EtOAc (300 mL×4). The combined organic layers were washed with brine, dried over Na2SO4, concentrated to give crude product. The crude product was purified by gel chromatography (petroleum ether to petroleum ether/EtOAc=5:1) to give 6-fluoro-4-nitroisobenzofuran-1(3H)-one (19.2 g, yield 79%) as a white solid. 1H-NMR (400 MHz, CDCl3) δ (ppm): 5.74 (s, 2H), 7.97-7.98 (dd, 1H), 8.24-8.27 (dd, 1H); LC-MS (ESI) m/z: 198(M+1)+
References:
US2010/35883,2010,A1 Location in patent:Page/Page column 1
697739-03-0
164 suppliers
$8.00/250mg

1207453-90-4
99 suppliers
$30.00/1g
33184-16-6
325 suppliers
$6.00/1g

1207453-90-4
99 suppliers
$30.00/1g
850462-64-5
104 suppliers
$11.00/250mg

1207453-90-4
99 suppliers
$30.00/1g