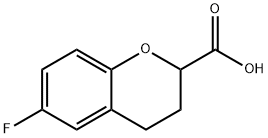
6-Fluorochromane-2-carboxylic acid synthesis
- Product Name:6-Fluorochromane-2-carboxylic acid
- CAS Number:99199-60-7
- Molecular formula:C10H9FO3
- Molecular Weight:196.18
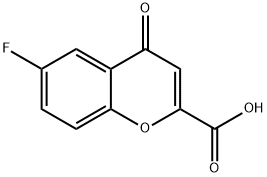
99199-59-4

99199-60-7
General procedure for the synthesis of 6-fluorobenzodihydropyran-2-carboxylic acid from 6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid: 30 g (0.144 mol) of 6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid, 5 g of wet palladium-carbon (5% Pd/carbon, 50% aqueous content) and 500 mL of glacial acetic acid were placed in an autoclave. After sealing three times under nitrogen protection, the nitrogen was replaced with hydrogen. Subsequently, the hydrogen was pressurized to 2.0 MPa and the reactor was heated to 70?80°C. During the reaction, when the hydrogen pressure in the kettle dropped, the hydrogen was promptly replenished to 2.0 MPa. The reaction was considered to be completed when the reactor pressure remained stable and did not change any more within half an hour. After the reaction was completed, the hydrogen in the autoclave was released and the reaction solution was filtered to recover the Pd/C catalyst. The filtrate was concentrated under reduced pressure to recover glacial acetic acid. The remaining concentrate was poured into 30 mL of petroleum ether and heated to give a white crystalline solid. The solid was filtered and dried to give a final 25 g of white 6-fluorobenzodihydropyran-2-carboxylic acid solid in 88.4% yield and 99.8% purity.

99199-59-4
145 suppliers
$34.46/1g

99199-60-7
248 suppliers
$70.68/5gm:
Yield:99199-60-7 90%
Reaction Conditions:
with palladium on activated charcoal;hydrogen;acetic acid at 75 - 80;Inert atmosphere;Autoclave;
Steps:
Preparation of 6-Fluoro-3, 4-dihydro-2H-1-benzopyran- 2-carboxylic acid (6):
Charged compound 5 (10.0 g) in 500 mL autoclave reactor and Pd/C (0.5 g) in acetic acid (150 ml) under nitrogen atmosphre, applied 15-20 kg Hydrogen pressure, and heated at 75-80 °C for 30-35 hrs. Complition of reaction was monitered over TLC. Cool, filter and washed with acetic acid 25 ml. Concentrate the reaction mass under reduced pressure. Charge toluene in deggased mass and aq. NaOH Solution (2.28g in 30 ml water), stir for 30 min and separated aq. and toluene layer. Aq. layer acidified with conc. HCl to get solid compound. Filter, washed with water and dry to get compound 8.47 g (6). 6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid (6) Yield: 90.00 %, Colour: Light yellow Solid, Molecular Formula: C10H9FO3, Mol. Wt.: 196.17g mol-1, Elemental composition (%): Calculated: C, 61.22; H, 4.62; F, 9.68; O, 24.47, Found: C, 61.28; H, 4.58; F, 9.66; O, 24.48, 1H NMR (400 MHz, CDCl3, ppm): δ = 8.61(Bs, 1H, HO-Acid), 6.76-7.01(m, 3H, H-Ar), 4.78(t, 1H, Methine), 2.85-2.82(t, -CH2-CH2-Ar, J=12 Hz), 2.37-2.14(m, 2H, -CH2-CH2-Ar), 13C NMR (400 MHz, CDCl3, ppm): δ = 175.2, 158.4, 156.0, 149.0, 122.4-122.3, 117.9-117.8, 115.5-115.2 73.2, 24.1-23.5, MS (ESI): m/z 196.18 (M).
References:
Dongare, Balasaheb B.;Ghanwat, Anil A.;Kashid, Bharat B.;Khedkar, Vijay M.;More, Kishor R.;Salunkhe, Pravin H. [Bioorganic and medicinal chemistry letters,2020] Location in patent:supporting information
874649-82-8
94 suppliers
$9.00/250mg

99199-60-7
248 suppliers
$70.68/5gm:
371-41-5
533 suppliers
$8.00/25g

99199-60-7
248 suppliers
$70.68/5gm:
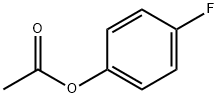
405-51-6
187 suppliers
$8.00/5g

99199-60-7
248 suppliers
$70.68/5gm:
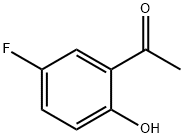
394-32-1
354 suppliers
$9.00/5g

99199-60-7
248 suppliers
$70.68/5gm: