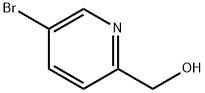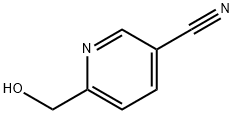
6-(HYDROXYMETHYL)NICOTINONITRILE synthesis
- Product Name:6-(HYDROXYMETHYL)NICOTINONITRILE
- CAS Number:31795-61-6
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
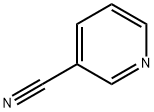
![3-Pyridinecarbonitrile, 6-[(acetyloxy)methyl]-](/CAS/20210305/GIF/31795-60-5.gif)
31795-60-5

31795-61-6
The general procedure for synthesizing 6-hydroxymethylpyridine-3-carbonitrile from the compound (CAS: 31795-60-5) was as follows: the compound of Example 31A (180 mg, 1.02 mmol) was dissolved in tetrahydrofuran (8 mL). Lithium hydroxide (48.94 mg, 2.04 mmol) was dissolved in water (5 mL) and this aqueous solution was added to the tetrahydrofuran solution. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the mixture was diluted with water and ethyl acetate. The aqueous phase was extracted three times with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over magnesium sulfate monohydrate, filtered and concentrated in vacuum. The resulting residue was used in the next reaction without further purification. Yield: 125 mg (91% yield). HPLC (Method 8): retention time (Rt) = 1.17 min. mass spectra (DCI): m/z 152 ([M + NH4]+). 1H-NMR (300 MHz, DMSO-d6): δ = 4.63 (d, 2H); 5.64 (t, 1H); 7.65 (d, 1H); 8.29 (dd, 1H); 8.92 (d, 1H) ppm.
![3-Pyridinecarbonitrile, 6-[(acetyloxy)methyl]-](/CAS/20210305/GIF/31795-60-5.gif)
31795-60-5
0 suppliers
inquiry

31795-61-6
77 suppliers
inquiry
Yield:31795-61-6 91%
Reaction Conditions:
with lithium hydroxide in tetrahydrofuran;water at 20; for 2 h;
Steps:
32A
The compound of Example31A (180 mg, 1.02 mmol) is dissolved in tetrahydrofuran (8 ml). Lithium hydroxide (48.94 mg, 2.04 mmol) is dissolved in water(5 ml) and added to the THF solution. The reaction is stirred for 2 hours at room temperature. The mixture is diluted with water and ethyl acetate. The aqueous phase is extracted three times with ethyl acetate. The organic phases are combined and washed with brine, dried with magnesium sulphate monohydrate, filtered and concentrated in vacuo. The residue is used without further purification. Yield: 125 mg(91% ofth.) HPLC (method 8): Rt= 1. 17 min MS(DCI) :m/z152 (M+NH4) +'H-NMR (300 MHz, DMSO-d6):8 = 4.63 (d, 2H);5. 64 (t, 1H); 7.65 (d, 1H); 8.29 (dd, 1H); 8.92 (d, 1H) ppm.
References:
BAYER HEALTHCARE AG WO2004/20412, 2004, A1 Location in patent:Page 47