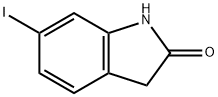
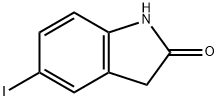
6-Iodoxindole synthesis
- Product Name:6-Iodoxindole
- CAS Number:919103-45-0
- Molecular formula:C8H6INO
- Molecular Weight:259.04
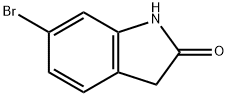
99365-40-9
300 suppliers
$6.00/1g

919103-45-0
61 suppliers
$111.00/100mg
Yield:919103-45-0 84%
Reaction Conditions:
with sodium iodide;copper(l) iodide;trans-N,N'-dimethylcyclohexane-1,2-diamine in 1,4-dioxane at 110; for 24 h;
Steps:
1
Preparation 1: 6-Iodooxindole A schlenk tube and stir bar were dried in an oven overnight and then were evacuated, filled with Ar(g) and cooled. The schlenk tube was charged with CuI (45 mg, 0.236 mmol, 5 mol %), 6-bromoxindole (1.0 g, 4.72 mmol), and NaI (1.42 g, 9.44 mmol). The schlenk tube was evacuated and backfilled with Ar(g) (3 times). Racemic trans-N,N'-dimethyl-1,2-cyclohexanediamine (74 μL, 0.472 mmol, 10 mol %) and anhydrous dioxane (4.72 mL) were added via syringe under Ar(g). The schlenk tube was sealed with a teflon valve and the suspension was stirred at 110° C. for 24 h. The reaction was then cooled to room temperature and 15% NH4OH(aq) (50 mL) was added to the reaction mixture while stirring. The suspension was allowed to stir for about 30 min after which the tan solid was vacuum filtered and dried affording 6-Iodooxindole in 84% yield (1.027 g, 3.96 mmol).
References:
US2007/15748,2007,A1 Location in patent:Page/Page column 5
88-73-3
8 suppliers
$14.00/25g

919103-45-0
61 suppliers
$111.00/100mg
41252-95-3
86 suppliers
$21.00/100mg

919103-45-0
61 suppliers
$111.00/100mg
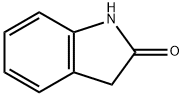
59-48-3
457 suppliers
$5.00/1g

919103-45-0
61 suppliers
$111.00/100mg
193354-13-1
68 suppliers
$29.00/100mg