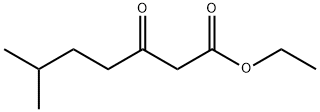
6-METHYL-3-OXO-HEPTANOIC ACID ETHYL ESTER synthesis
- Product Name:6-METHYL-3-OXO-HEPTANOIC ACID ETHYL ESTER
- CAS Number:57689-16-4
- Molecular formula:C10H18O3
- Molecular Weight:186.25
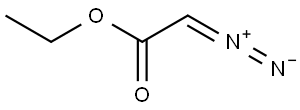
623-73-4
151 suppliers
$27.30/25ML
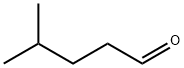
1119-16-0
75 suppliers
$133.00/100mg

57689-16-4
18 suppliers
$45.00/10mg
Yield:57689-16-4 55%
Reaction Conditions:
with tin(ll) chloride in dichloromethane at 25; for 1.16667 h;
Steps:
6.b
b) 6-Methyl-3-oxo-heptanoic acid ethyl ester A solution of diazo-acetic acid ethyl ester (555 mg, 4.86 mmol) in dichloromethane (8 mL) was treated with tin (II) chloride (88 mg, 0.463 mmol) and stirred vigorously at 25° C. A solution of 4-methyl-pentanal (464 mg, 4.63 mmol) in dichloromethane (2 mL) was added to the mixture dropwise over 10 min resulting in gas evolution. After the nitrogen evolution ceased (approximately 1 h), the reaction was extracted with brine and diethyl ether (2*80 mL). The organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo. The crude material was purified by flash column chromatography (ISCO RediSep column, 0 to 7% ethyl acetate in hexanes) to afford the desired product, 6-methyl-3-oxo-heptanoic acid ethyl ester (478 mg, 55%), as a light yellow oil. 1H NMR (400 MHz, CDCl3) δ: 0.89 (6H, d, J=6.3 Hz), 1.26-1.29 (4H, m), 1.48-1.57 (2H, m), 2.53 (2H, t, J=7.4 Hz), 3.42 (2H, s), 4.18 (2H, quartet, J=7.6 Hz).
References:
US2009/62263,2009,A1 Location in patent:Page/Page column 29
513-38-2
188 suppliers
$62.00/25mL

141-97-9
826 suppliers
$5.00/25g

57689-16-4
18 suppliers
$45.00/10mg
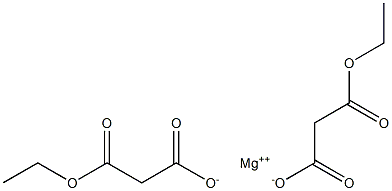
37517-78-5
27 suppliers
inquiry

57689-16-4
18 suppliers
$45.00/10mg
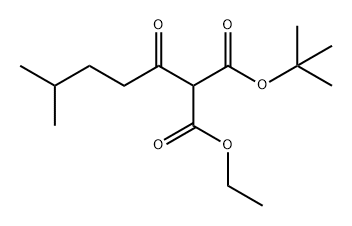
80572-74-3
0 suppliers
inquiry

57689-16-4
18 suppliers
$45.00/10mg
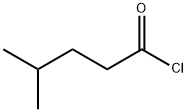
38136-29-7
141 suppliers
$75.00/2.5g

57689-16-4
18 suppliers
$45.00/10mg