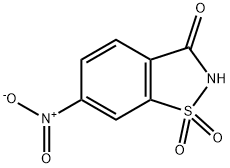
6-Nitro-1,2-benzisothiazolin-3-one 1,1-dioxide synthesis
- Product Name:6-Nitro-1,2-benzisothiazolin-3-one 1,1-dioxide
- CAS Number:22952-24-5
- Molecular formula:C7H4N2O5S
- Molecular Weight:228.18
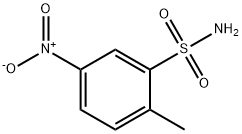
6269-91-6

22952-24-5
General procedure for the synthesis of 6-nitro-1,2-benzisothiazolin-3-one 1,1-dioxide from 2-methyl-5-nitrobenzenesulfonamide: A solution of chromium trioxide (20.4 g, 0.204 mol) in water (150 mL) was added drop-wise to concentrated sulfuric acid (190 mL) under ice-bath conditions. Subsequently, 2-methyl-5-nitrobenzenesulfonamide (10 g, 46.3 mmol) was added and the reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the mixture was filtered and the residue was washed with water and then dissolved in sodium bicarbonate solution and filtered again under vacuum. The filtrate was acidified with concentrated hydrochloric acid to precipitate. The precipitated product was collected by filtration and washed with plenty of cold water. Finally, the product was dried in a vacuum oven at 40°C to give a white powder in 72% yield. The melting point of the product was 205.6-205.8 °C (literature value: 205-207 °C); IR spectra showed characteristic absorption peaks located at 3103, 1720, 1608, 1541, 1354, 1340, 1184, 1128 cm-1 ; 1H NMR (DMSO-d6, 300 MHz) δ/ ppm: 8.02 (1H, d, J = 8.5 Hz), 8.56 (1H, d, J = 8.2 Hz), 8.71 (1H, s), 10.2 (1H, br, NH); 13C NMR (DMSO-d6, 75 MHz) δ/ ppm: 117.2, 126.4, 129.8, 135.2, 143.0, 151.7, 162.2.

6269-91-6
150 suppliers
$9.00/5g

22952-24-5
81 suppliers
$7.00/1g
Yield: 72%
Reaction Conditions:
with chromium(VI) oxide;sulfuric acid in water at 20; for 24 h;
Steps:
4.2.3. 6-Nitrosaccharin (4)
To solution chromium trioxide (20.4 g, 0.204 mol) in water (150 mL) was added concentrated sulfuric acid (190 mL) dropwise in the ice bath. 2-Methyl-5-nitrobenzenesulfonamide (10 g, 46.3 mmol) was added and the mixture was stirred at room temperature for 24 h. The mixture was filtered and the residue washed with water, dissolved in sodium bicarbonate solution and filtered under vacuum. The filtrate was acidified with concentrated HCl. After the precipitated product was filtered and washed with abundant cold water, it was dried in the vacuum-oven at 40 °C. The product was obtained a white powder in 72% yield. Mp 205.6-205.8 °C, (lit.36 205-207 °C); IR: 3103, 1720, 1608, 1541, 1354, 1340, 1184, 1128 cm-1; 1H NMR (DMSO-d6, 300 MHz) δ/ppm: 8.02 (1H, d, J = 8.5 Hz), 8.56 (1H, d, J = 8.2 Hz), 8.71 (1H, s), 10.2 (1H, br, NH); 13C NMR (DMSO-d6, 75 MHz) δ/ppm: 117.2, 126.4, 129.8, 135.2, 143.0, 151.7, 162.2.
References:
Gener, Nahit;Demir, Dudu;Sonmez, Fatih;Kucukislamoglu, Mustafa [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 9,p. 2811 - 2821] Location in patent:experimental part
154712-47-7
13 suppliers
inquiry

22952-24-5
81 suppliers
$7.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

22952-24-5
81 suppliers
$7.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

22952-24-5
81 suppliers
$7.00/1g
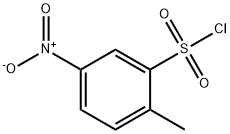
121-02-8
165 suppliers
$6.00/1g

22952-24-5
81 suppliers
$7.00/1g